Figure 2.
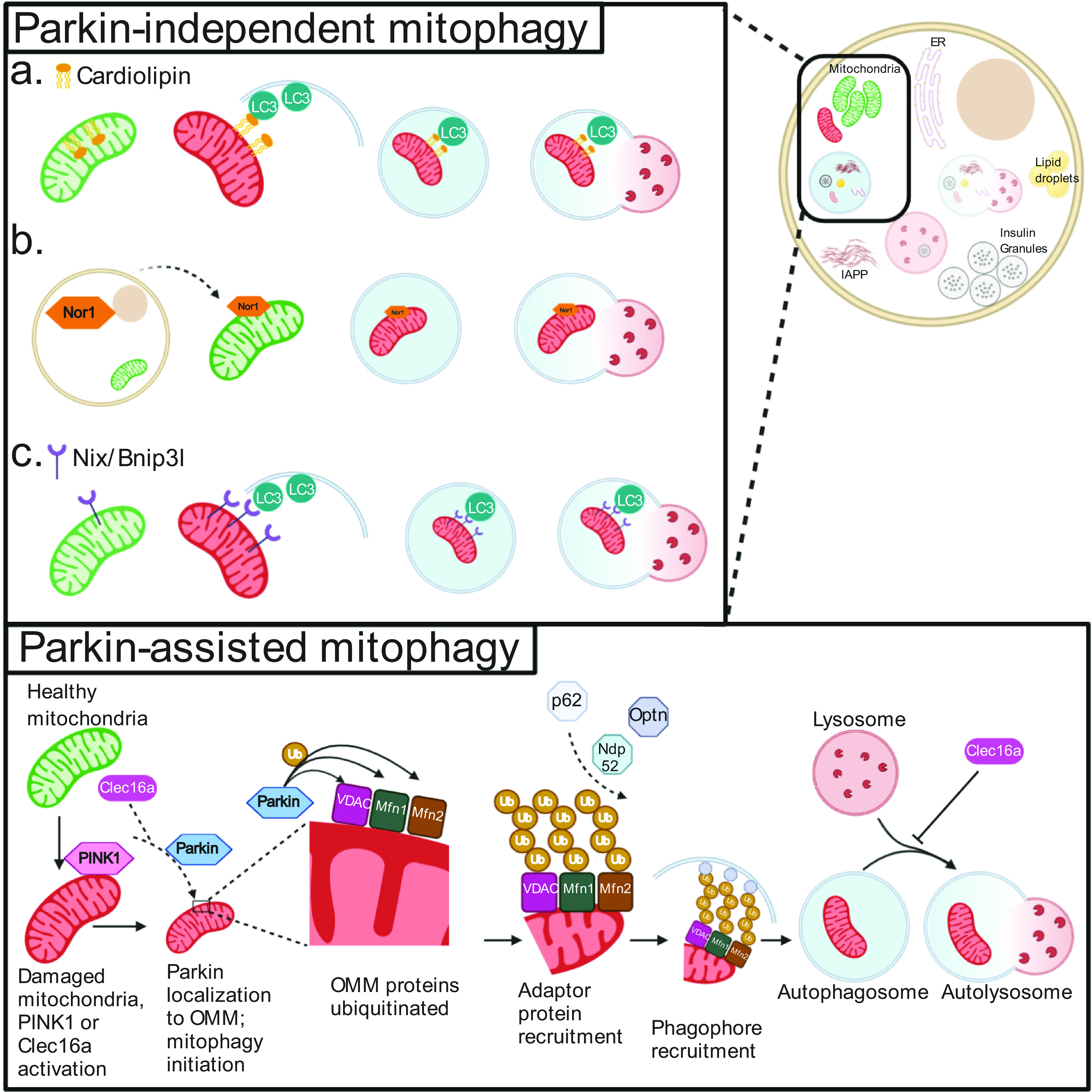
Mitophagy pathways in β-cells. Mitophagy can be initiated by a number of mechanisms, including those dependent (bottom) or independent (top) of the E3 ubiquitin ligase Parkin. During Parkin-mediated mitophagy, damaged mitochondria are recognized by activation of the Clec16a-Nrdp1-Usp8 complex, which leads Parkin to translocate to the OMM. Here, Parkin ubiquitinates a number of membrane proteins (including but not limited to VDAC, Mfn1, and Mfn2). The presence of ubiquitin (Ub) complexes signals to adaptor proteins capable of binding Ub and phagophore/autophagosome membrane resident proteins to traffic mitochondria to autophagosomes. Once the mitochondria are targeted to the phagophore, subsequent closure of the autophagosome and fusion with lysosomes result in selective mitochondrial clearance. These final steps are common to both Parkin-dependent and -independent mitophagy, as can be seen in a–c (top panel). Parkin-independent pathways of mitochondrial cargo recognition and clearance include the following (top panel): flipping of the phospholipid cardiolipin to the OMM, which can bind directly to LC3 on the expanding phagophore and is potentially targeted by the mitochondrial targeted tetrapeptide SS-31 (a); nuclear orphan receptor Nor1 translocation to the mitochondria (b); and increased expression of transmembrane receptor Nix/BNIP3L, which can again directly bind LC3 (c).
