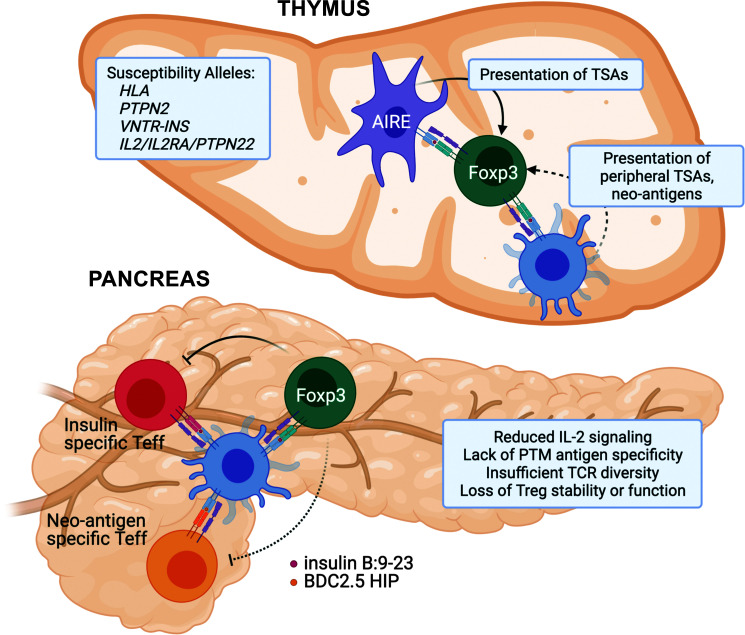
Figure 1.
Putative mechanisms underlying Foxp3+ Treg dysfunction in T1D. Thymus: Genetic susceptibility in T1D points to several pathways that are important for regulatory T-cell development, including TCR activation (HLA/PTPN22) and IL-2 signaling pathways (IL2/IL2RA). Variable number of tandem repeat polymorphisms upstream of the insulin promoter (VNTR-INS) may impact thymic transcript levels of insulin, while peripheral TSAs are presented to developing thymocytes by mTECs and DCs. Although DCs can migrate from periphery and have the potential to bring novel posttranslationally modified (PTM) self-antigens (neo-antigens), the process is likely inefficient, leading to escape of neo-antigen reactive autoimmune T cells and lack of development of neo-antigen reactive Tregs. Pancreas: Although we are lacking data regarding human Treg function in the pancreas during T1D development, studies in NOD mice have defined several potential issues that lead to Treg dysfunction in autoimmune diabetes. At the pancreatic tissue site, Tregs suffer from insufficient activation of the IL-2 signaling pathway, reduced TCR repertoire and diversity, and progressive loss of function as a result of chronic exposure to inflammatory mediators. Teff, effector T cell.