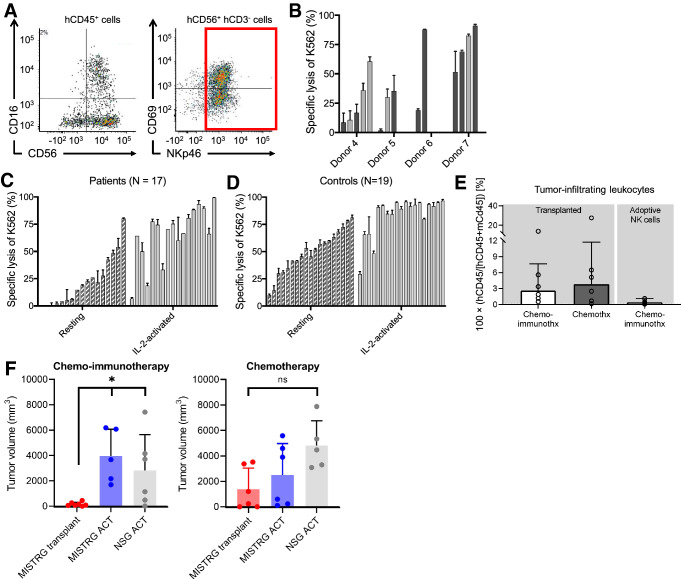
Fig. 2.
Functional evaluation of NK cells from MISTRG mice, patients with neuroblastoma, and healthy controls. a Gating strategy of FACS-isolation of CD3− CD56+ NKp46+ NK cells. b FACS-isolated NK cells from the spleens of transplanted MISTRG mice were stimulated with IL-2 (50 IU/mL) for 12 to 18 h and tested in a natural killer cytotoxicity assay against K562 cells. Each bar represents one animal. Tumor-bearing mice are marked by dark grey and tumor-free animals (because of tumor engraftment failure) by light grey bars. c Ex vivo lysis of K562 cells by peripheral blood NK cells from 17 patients with newly diagnosed and untreated neuroblastoma and d 19 healthy adult controls. e Residual tumor-infiltrating human CD45+ cells at the end of therapy are shown for mice with endogenous human hematopoiesis and contrasted to mice that received adoptive NK cell injections. Transplanted animals had significantly more infiltrating cells (P = 0.01; Mann–Whitney test). f Experimental cohorts were grouped by intervention (chemo-immunotherapy and chemotherapy) and separated by the
source of effector cells. Transplanted MISTRG mice (red) are compared with MISTRG (blue) and NSG (grey) mice that received ACT of NK cells from one donor. Among mice receiving chemo-immunotherapy, transplanted MISTRG mice (N = 7) had more significant tumor growth suppression than did MISTRG and NSG mice that received adoptive NK cells with therapy (N = 5 and 6; P = 0.003; one-way ANOVA). No significant differences occurred between these groups treated with chemotherapy (each MISTRG groups N = 6, NSG group N = 5)