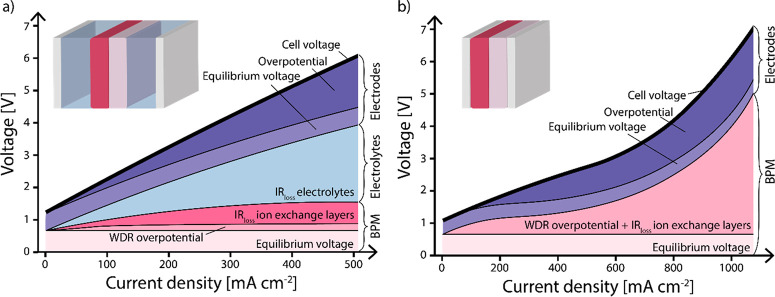
Figure 2.
Voltage distribution of an electrochemical cell (a) in a liquid–liquid environment with extreme pH (favoring equilibrium voltage) and (b) in a zero gap configuration. Membrane voltage consists of equilibrium voltage, water dissociation reaction (WDR) overpotential, and ion-exchange layers (data obtained from Chen et al., 2020, for (a)36 and Shen et al., 2017, for (b),37 where no data was available to discriminate the WDR overpotential from ohmic losses of the ion-exchange layers). The membrane contributions, together with the ohmic losses of the electrolytes (if applicable) and voltage of the electrodes, result in the cell voltage.