Abstract
Background:
Insulin, leptin, and adiponectin regulate energy balance and may influence infant growth via their presence in human milk. Maternal body mass index has been associated with human milk insulin, leptin, and adiponectin concentrations, but results are inconsistent. Maternal serum hormone concentrations and fat mass may better characterize human phenotype and be more appropriate predictors of human milk insulin, leptin, and adiponectin.
Research aim:
To examine the associations of human milk insulin, leptin, and adiponectin with their concentrations in maternal circulation and with maternal fat mass.
Methods:
Insulin, leptin, and adiponectin were measured in serum and human milk at 1 month postpartum in N = 25 women. Total body fat mass and fat-free mass were measured using bioelectrical impedance analysis. Linear regression modeling was used to examine associations of serum hormone concentrations or fat mass with human milk insulin, leptin, and adiponectin after adjusting for covariates.
Results:
Serum insulin (p = .007), leptin (p < .001), and adiponectin (p < .001) were each associated with their respective concentrations in human milk. Fat mass was positively associated with insulin (p = .005) and leptin (p < .001), but not with adiponectin (p = .65), in human milk.
Conclusions:
Human milk insulin, leptin, and adiponectin were positively associated with their concentrations in serum, and human milk insulin and leptin were associated with maternal fat mass. Future research is needed to elucidate the role of human milk hormones in infant energy balance and growth.
Background
The prevalence of obesity among pregnant women continues to rise (Chen et al., 2018). Globally, an estimated 1 in 8 women of childbearing age had obesity in 2015 (Global Burden of Disease Study 2015 Obesity Collaborators, 2017). Obesity during pregnancy increases the risk for perinatal complications, as well as the child’s long-term risk for obesity and chronic disease (Catalano & Shankar, 2017). In a meta-analysis, children with obesity were three times more likely to have been born to a mother with obesity compared to normal weight children (Heslehurst et al., 2019). Thus, it is important for public health that we understand the mechanisms by which maternal obesity increases the risk for obesity in the child.
Pregnancy is a critical period for programming children’s metabolism in a manner that influences their long-term risk for obesity, diabetes, and cardiovascular disease (Barker, 1990; Padmanabhan et al., 2016; Woo Baidal et al., 2016). Infancy is also a critical period for programming obesity risk. Researchers have consistently shown that relatively rapid weight gain during infancy is associated with an increased risk for obesity in childhood and beyond (Zheng et al., 2018). As human milk is the main source of nutrition during early infancy, it may play a role in programming obesity (i.e. “lactational programming”). Researchers have used cross fostering studies in animals (Gorski et al., 2006; Oben et al., 2010; Reifsnyder et al., 2000), as well as a banked human milk study (Plagemann et al., 2002; Rodekamp et al., 2005), to provide evidence that there is a role for human milk in programming obesity risk that is independent of the risk associated with the intrauterine environment. To date, it is unclear how human milk exerts an influence on infant growth and long-term obesity risk.
Human milk is a dynamic biological fluid that varies considerably among mothers. Human milk contains insulin, leptin, and adiponectin, which are hormones that play a role in the regulation of energy balance, appetite, and glucose and fat metabolism in adults (Petersen & Shulman, 2018; Scheja & Heeren, 2019), and are hypothesized to contribute to the development of infant appetite regulation and body composition via human milk (Badillo-Suárez et al., 2017). Concentrations of these hormones in human milk have been associated with infant weight and lean mass in some studies, but the direction and magnitude of these associations is inconsistent (Badillo-Suárez et al., 2017). Thus, it is unclear whether exposure to these hormones in human milk protects against future obesity by promoting satiety and preventing rapid infant weight gain or conversely, whether it predisposes the infant to later metabolic dysfunction and increases long-term obesity risk (Badillo-Suárez et al., 2017). Nonetheless, insulin, leptin, and adiponectin are dysregulated with obesity (Scheja & Heeren, 2019) and their presence in human milk suggests that they may play a role in the transmission of obesity from mother to child.
Maternal phenotype, behavior (e.g., dietary intake), and environment may account for some of the individual variation in human milk composition (Fields et al., 2016). Specifically, human milk leptin has been consistently and positively associated with maternal body mass index (BMI; Brunner et al., 2015; Fields et al., 2017; Sadr Dadres et al., 2019; Young et al., 2017). However, the direction and magnitude of the association of maternal BMI with human milk insulin and adiponectin has been inconsistent (Chan et al., 2018; Fields et al., 2017; Sadr Dadres et al., 2019; Shehadeh et al., 2003; Young et al., 2017; Yu et al., 2018). It is possible that some of this inconsistency is due to the fact that may researchers have used BMI in early pregnancy or at the time of milk collection, which is problematic because BMI is not a reliable estimate of adiposity (Gába & Přidalová, 2016).
Researchers have also found evidence that concentrations of insulin, leptin, and adiponectin in human milk reflect their concentrations in maternal circulation (Fakhreldin, 2018; Jovanovic-Peterson et al., 1989; Young et al., 2017). It has been well-established that fat mass (FM) is more closely related to circulating insulin, leptin, and adiponectin concentrations than BMI (Goossens, 2017). Thus, maternal serum hormone concentrations and measures of FM may better characterize the metabolic phenotype of the mother and be more appropriate predictors of human milk insulin, leptin, and adiponectin. However, most evidence that maternal metabolic phenotype affects human milk composition comes from researchers that have compared the human milk of mothers with and without diabetes (Jovanovic-Peterson et al., 1989; Whitmore et al., 2012; Yu et al., 2018). Thus, we need more information on the associations of insulin, leptin, and adiponectin concentrations in maternal circulation with their concentrations in human milk, particularly among non-diabetic mothers.
In this study, we aimed to examine the associations of human milk insulin, leptin, and adiponectin with their concentrations in maternal circulation and with maternal FM. We hypothesized that human milk insulin, leptin, and adiponectin concentrations would be positively associated with their concentrations in maternal serum and with maternal adiposity.
Methods
Design
A cross-sectional, observational study of mothers at 1-month postpartum was used to address our research aim. We chose 1-month postpartum to avoid the potential confounding effect of rapid fluid losses during the early postpartum period on the measurement of maternal FM, to ensure lactation was established, and to capture women before returning to work (Lukaski et al., 2007). The Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB) reviewed and approved all study procedures and amendments.
Setting
The cohort comprised women living near Birmingham, Alabama, an urban area in the southeast United States. The ethnic composition in this area is approximately 50% non-Hispanic white, 44% Black or African American, and 4% Hispanic or Latino (United States Census Bureau, n.d.). Alabama has one of the lowest breastfeeding rates in the United States, with approximately 68% of women ever breastfeeding and 21% exclusively breastfeeding at 6 months (Centers for Disease Control and Prevention, 2018). We recruited exclusively breastfeeding mothers for this study through flyers placed at local prenatal care classes, obstetrics offices, pediatricians’ offices, daycare centers, and online in a local breastfeeding support group between January 2017 and March 2018.
Sample
We screened interested mothers via telephone for these initial inclusion criteria: 2–4 weeks postpartum, ≥ 19 years of age, body weight < 300 kg (capacity of study scale), and exclusively breastfeeding (i.e., no formula supplementation) their singleton infant; and exclusion criteria: diagnosed type 1 or type 2 diabetes mellitus, presence of any medical condition that could affect hormone levels (i.e., thyroid disorder, polycystic ovarian syndrome, diseases of the adrenal or parathyroid gland) or significantly interfere with breastfeeding, reported use of any medications believed to affect hormone levels, self-reported any nicotine or illicit drug use during pregnancy or while lactating, inability to understand and communicate in verbal and written English, infant diagnosis of failure to thrive or any genetic or congenital defect or medical condition known to interfere with growth and development. We screened a total of 86 women over the 14 months of recruitment. Of those screened, 25 participants were eligible and enrolled in the study. Based on previously published data (Chan et al., 2018; Fakhreldin, 2018; Quinn et al., 2015; Young et al., 2017), we anticipated a medium to large effect size (0.4–0.72) for associations of maternal serum hormones and FM with human milk insulin, leptin, and adiponectin. Using multiple linear regression with 3 predictor variables and assuming an effect size of 0.4, we would have 85% power to detect a significant association with a sample size of 25. Power calculations were conducted using G*Power 3.1.9.7. (Faul et al., 2007) and assuming a significance level of .05.
Measurements
We asked participants to report their age, race-ethnicity (categorized as non-Hispanic White, non-Hispanic Black, Hispanic or Latino, Native American or American Indian, or Asian/Pacific Islander), marital status (categorized as married or living as married, never married, separated, divorced, or widowed), parity, pre-pregnancy weight, weight gained during gestation, gestational age at delivery, infant birth weight, and household income via questionnaire.
We measured maternal FM and fat-free mass (FFM) using segmental multifrequency bioelectrical impedance analysis (BIA; seca mBCA, seca gmbh & co. kg, Hamburg, Germany), which has been validated using the 4-compartment model with a correlate of over 98% for FFM (Bosy-Westphal et al., 2013). Body mass was measured to the nearest 45 g with participants wearing minimal clothing and no shoes, and FM was calculated as the difference between total body mass and FFM. Height was measured to the nearest 1 mm using a seca® 264 digital stationary stadiometer (seca gmbh & co. kg, Hamburg, Germany).
Fasting blood samples were immediately processed to extract serum. Serum was stored at −80°C until analysis. Insulin in serum was measured by immunofluorescence on a TOSOH 900 AIA analyzer (TOSOH Bioscience, South San Francisco, CA). Mean intra- and inter-assay coefficients of variation (CVs) were 1.49% and 3.95%, respectively. Serum leptin was measured by radioimmunoassay (Millipore, Billerica, MA) in one assay with an intra-assay CV of 7.27%. Serum adiponectin was measured by radioimmunoassay (Millipore, Billerica, MA) using one assay with a mean intra-assay CV of 0.83%.
Participants reported the time and from which breast they last fed or expressed milk. We measured the weight of the container (e.g., bottle) used for the human milk collection prior to and again immediately following the milk expression to the nearest 1 g on an Ohaus CS2000 electronic scale (Ohaus, Parsippany, NJ). The total amount of expressed milk collected was calculated as the difference in the container weight pre- and post-milk expression. Human milk samples were thoroughly mixed and divided into 3-ml aliquots. Aliquots were stored at −80°C until analyses. For analyses, human milk aliquots were thawed on ice and milk fat was separated from the aqueous phase by centrifugation (3,000 xg for 10 min). Assays were all conducted the same day on the newly skimmed human milk. Insulin in human milk was measured by immunofluorescence on a TOSOH 900 AIA analyzer (TOSOH Bioscience, South San Francisco, CA) with mean intra- and inter-assay CVs of 1.90% and 2.55%, respectively. Leptin was measured by immunoassay (Human Leptin Quantikine ELISA Kit; R&D Systems, Minneapolis, MN) with mean intra- and inter-assay CVs of 4.48% and 13.56%, respectively. Adiponectin was measured by radioimmunoassay (Millipore, Billerica, MA) using one assay with a mean intra-assay CV of 7.67%.
Of the 25 participants enrolled in the study, one participant had given birth prior to 37 weeks gestation to an infant weighing less than 2,500 g. Exclusion of this participant’s data did not change results; therefore, her data were included in the final analyses. One participant was a statistical outlier for human milk insulin, even after log transformation, and reported breastfeeding an older child (in addition to the exclusively breastfed infant included in our study), and so her data were excluded from all human milk insulin analyses. Excluding this participant did not change the results of the human milk leptin or adiponectin models and so, she was included for these analyses.
Data Collection
All data were collected between February 2017 and March 2018. Written informed consent was obtained from all participants who chose to enroll in the study (M = 26.0 days postpartum, SD = 4.5 at enrollment). At least 24 hours after the enrollment visit, participants returned for the assessment visit (M = 33.3 days postpartum, SD = 4.4). This was done to comply with Institutional Review Board requirements that study participants be given at least 24 hours to consider study participation and the opportunity to withdraw after reviewing and receiving a copy of the consent form. All data were entered into and stored in a password-protected, secure database. Consent forms and original copies of data collection forms were stored in a locked filing cabinet within a locked research lab to guard confidentiality. For the assessment visit, participants arrived between 0800 and 1000 following an overnight fast (no food or drink except for plain water) of at least 10 hours. As previously described (Wingo et al., 2018), we asked participants to refrain from drinking alcohol within 24 hours of the assessment visit, to avoid exercise or sauna use within 12 hours of the assessment visit, and to refrain from using hand or body lotion the morning of the assessment visit. Immediately upon arrival at the assessment visit, we collected a fasting venous blood sample from each participant. Subsequently, with participants still fasted, a sample of human milk was collected. In-clinic sample collection reduced the potential that diurnal variation would influence human milk hormone concentrations. The entire contents of the breast opposite the one last used for a feed or expression were collected using the participant’s personal breast pump. A study investigator was in the room with participants to confirm that no more milk was coming from the breast at the completion of the expression, which ensured each sample contained fore-, mid-, and hind-milk. Immediately following the human milk collection, height, weight, and body composition (total FM and FFM) measurements were taken.
Data Analysis
All data are presented as means and standard deviations, unless otherwise specified. All statistical tests were two-tailed. We used Wilcoxon signed-rank non-parametric tests to compare insulin, leptin, and adiponectin concentrations in human milk with their concentrations in maternal serum.
We performed exploratory analyses of the following potential covariates using stepwise linear regression to obtain the most parsimonious model: gestational weight gain, age, parity, household income, infant gender, infant age at milk collection, and time since last breastfeeding to variability in human milk insulin, leptin, and adiponectin concentrations. We used separate multiple linear regression models to examine the association of maternal FM, adjusted for FFM to account for overall body size, or serum hormone concentration with human milk insulin, leptin, and adiponectin. We adjusted final regression models predicting human milk insulin for the time since the last breastfeeding, we did not adjust final regression models for human milk leptin for any additional covariates, and we adjusted final regression models predicting human milk adiponectin for maternal age at measurement. We log-transformed serum and human milk analyte concentrations for regression analyses. For all models, residuals were normally distributed with a mean of 0 and standard deviation of 1. Alpha was set at .05 for statistical significance and all analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, North Carolina).
Results
Characteristics of the Sample
Characteristics of the study population are described in Table 1. The mean (SD) amount of expressed milk collected was 97.3 g (66.8). Participants were predominately White (Non-Hispanic; 96%, n = 24) and reported that they were married or living as married (96%, n = 24). According to BMI at the assessment visit, 4% (n = 1) of the study sample were underweight (BMI < 18.5), 32% (n = 8) were normal weight (18.5 ≤ BMI < 25.0), 20% (n = 5) were overweight (25.0 ≤ BMI < 30.0), 44% (n = 11) were obese (BMI ≥ 30.0). None of the participants reported having gestational diabetes during their pregnancy. Just over half of participants had a male infant (56%, n = 14) and there were no differences by infant gender in human milk concentrations of insulin, leptin, or adiponectin (not shown).
Table 1.
Characteristics of the Study Cohort (N = 25)
| Variable | M (SD) | Range |
|---|---|---|
| Age (years) | 31.3 (4.4) | 20.9–38.0 |
| Pre-pregnancy BMI (kg/m2) | 27.7 (7.7) | 17.5–43.9 |
| Gestational age at delivery (weeks) | 39.8 (1.5) | 34.0–42.0 |
| Gestational weight gain (kg)a | 14.9 (5.5) | 0.0–23.6 |
| Infant birth weight (g) | 3553.2 (561.6) | 2182.9–4535.9 |
| Maternal BMI (kg/m2) | 29.4 (7.2) | 18.3–45.2 |
| Maternal total fat mass (kg) | 32.9 (14.8) | 12.8–71.8 |
| Maternal fat-free mass (kg) | 47.3 (7.1) | 36.4–66.1 |
Note. M (SD), Mean (Standard Deviation); BMI, body mass index.
n = 24, one participant could not recall amount of gestational weight gain.
Concentrations of Insulin, Leptin, and Adiponectin in Serum and Human Milk
Analyses comparing concentrations of insulin, leptin, and adiponectin in serum with their respective concentrations in human milk are shown in Figure 1.
Figure 1.
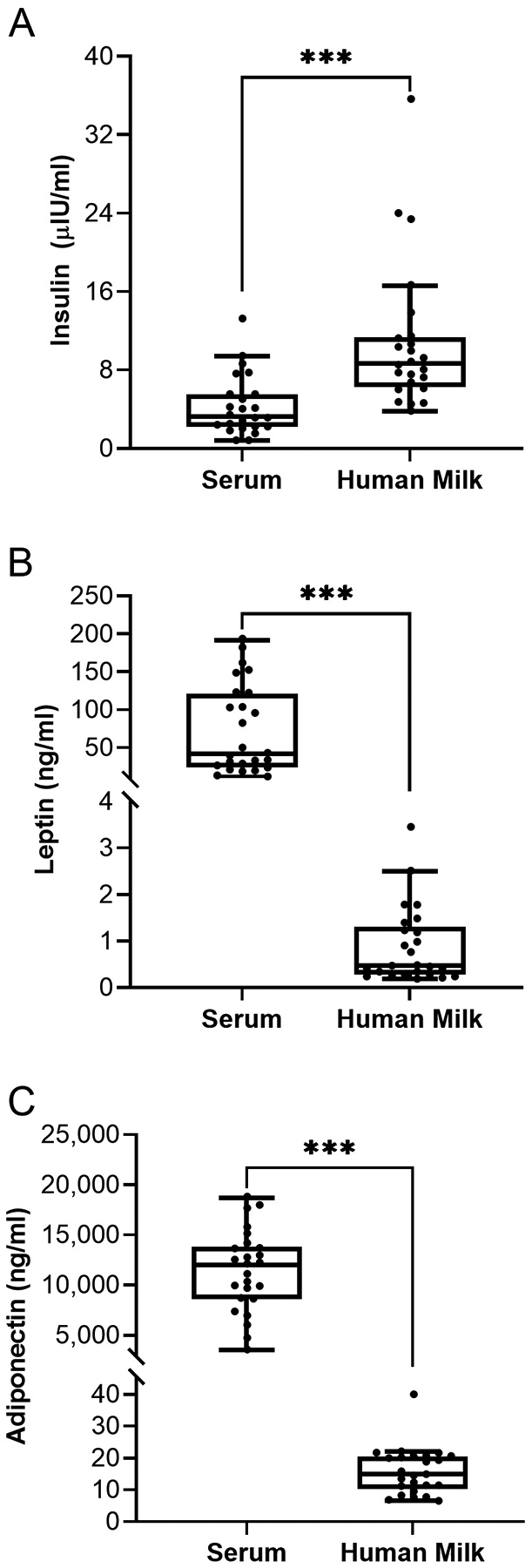
Wilcoxon Signed-Rank Tests for the Comparison of Maternal Serum and Human Milk Hormone Concentrations at 1 Month Postpartum. Note. The center line of the box is the median (Mdn), the limits of the box are the 25th and 75th percentile (interquartile range [IQR]), and whiskers are 1.5 times the IQR below and above the 25th and 75th percentiles, respectively. Each dot represents an individual participant. Panel A: Serum versus human milk insulin (Mdn [IQR]: 3.25 μIU/ml [3.30] vs. 8.65 [4.90], respectively). Panel B: Serum versus human milk leptin (Mdn [IQR]: 41.75 ng/ml [95.29] vs. 0.47 [0.94], respectively). Panel C: Serum versus human milk adiponectin (Mdn [IQR]: 12,030.00 ng/ml [4,970.00] vs. 14.90 [9.19], respectively). ***p < .001.
Serum with Human Milk Insulin, Leptin, and Adiponectin
Results of regression models for associations of serum insulin, leptin, and adiponectin with their respective concentrations in human milk are shown in Table 2.
Table 2.
Standardized Regression Coefficients of Associations Between Serum Hormone (Insulin, Leptin, or Adiponectin) Concentration or Fat Mass and Human Milk Hormone (Insulin, Leptin, or Adiponectin) Concentration (N = 25)
| Model | Outcome | Predictor | b* | t | p |
|---|---|---|---|---|---|
| 1 | Human milk insulina | Serum insulin, adjustedb | 0.50 | 2.97 | <.01 |
| 2 | Human milk insulina | Fat mass, adjustedc | 1.13 | 3.13 | <.01 |
| 3 | Human milk leptin | Serum leptin | 0.96 | 17.26 | <.0001 |
| 4 | Human milk leptin | Fat mass, adjustedd | 1.02 | 5.45 | <.0001 |
| 5 | Human milk adiponectin | Serum adiponectin, adjustede | 0.77 | 6.46 | <.0001 |
| 6 | Human milk adiponectin | Fat mass, adjustedf | 0.20 | 0.46 | .65 |
n = 24, one mother was a statistical outlier for human milk insulin and so her data were excluded from all milk insulin analyses.
Linear regression model was adjusted for duration of time from the last breastfeeding to the start of the human milk collection.
Linear regression model was adjusted for duration of time from the last breastfeeding to the start of the human milk collection and fat-free mass.
Linear regression model was adjusted for fat-free mass.
Linear regression model was adjusted for maternal age at measurement; n = 24, one mother was a statistical outlier and her data were excluded from this regression model.
Linear regression model was adjusted for maternal age at measurement and fat-free mass.
Adiposity with Human Milk Insulin, Leptin, and Adiponectin
Results of regression models for the association of maternal FM, adjusted for FFM, with human milk insulin, leptin, and adiponectin are shown in Table 2. Serum adiponectin was positively associated with human milk adiponectin concentrations (b* = 0.63, p < .001; not shown), but there was an outlier. Results excluding this outlier are shown in Table 2, Model 5.
Discussion
We expanded upon previous studies by showing that circulating insulin, leptin, and adiponectin were associated with their respective concentrations in human milk and that maternal adiposity at one month postpartum, measured with BIA, was associated with human milk insulin and leptin concentrations. Given the role of insulin, leptin, and adiponectin in the regulation of energy balance, this raises the possibility that human milk concentrations of these hormones play a role in the intergenerational transmission of obesity.
Our insulin results were consistent with previous research (Young et al., 2017). Given that women with obesity have higher circulating insulin concentrations (Kim et al., 2015), it is likely that the association of FM with human milk insulin reflects the association between circulating and human milk insulin. Although the importance of human milk insulin for the infant is unclear, the higher insulin concentration in human milk versus serum may be important to help account for the immature secretory function of the infant pancreatic beta cells (Bonner-Weir et al., 2016; Henquin & Nenquin, 2018). Young et al. (2017) found that human milk insulin levels decreased across lactation, which would be consistent with the pancreatic beta cell maturation and improved glucose-stimulated insulin secretion that occurs across infancy (Bonner-Weir et al., 2016; Henquin & Nenquin, 2018). Additionally, it is possible that human milk insulin changes in response to the availability of energy from the mother, and thereby may support infant appetite regulation. In support of this, Jovanovic-Peterson et al. (1989) demonstrated that human milk insulin concentrations rose in response to an insulin infusion in diabetic mothers. It will be of interest in the future to better characterize how closely insulin in human milk aligns with that in circulation, and whether human milk insulin concentrations change across the day in response to maternal dietary patterns.
Our results for leptin are consistent with evidence that leptin in the aqueous portion of the human milk is derived from maternal circulation (Casabiell et al., 1997; Houseknecht et al., 1997), and, as it is primarily secreted by adipocytes, circulating leptin concentrations reflect adiposity (Scheja & Heeren, 2019). Human milk leptin may influence infant appetite control through its actions on the infants developing hypothalamic circuitry (Badillo-Suárez et al., 2017). However, additional research is needed to understand the importance of leptin for infant development and health.
Other researchers have reported positive associations between serum and human milk adiponectin concentrations (Savino et al., 2012; Weyermann et al., 2006; Young et al., 2017), but the origin of human milk adiponectin is unclear (Weyermann et al., 2006). Despite the well-established inverse association between overall adiposity and circulating adiponectin (Scheja & Heeren, 2019), the association of maternal adiposity with human milk adiponectin remains unclear. Some researchers have reported positive associations between maternal BMI and human milk adiponectin (Young et al., 2017; Yu et al., 2018). While other researchers have found no association (Chan et al., 2018; Weyermann et al., 2007) or a negative association (Sadr Dadres et al., 2019) between maternal BMI and human milk adiponectin. These inconsistent findings may be due to changes in the association of maternal BMI with human milk adiponectin across the postpartum period (Sadr Dadres et al., 2019; Young et al., 2017) or to the possibility that human milk adiponectin may be more closely related to pre-pregnancy BMI versus current BMI (Bronsky et al., 2006). Ultimately, we need more research to understand factors aside from serum adiponectin that influence human milk adiponectin concentrations, as this could have important implications for the infant.
Limitations
Our study had several limitations. Generalizability is minimal due to the small, predominantly White cohort within a select geographic region. The lack of objective data prior to or during pregnancy prevented us from being able to examine and compare associations of pre-pregnancy BMI and gestational weight gain with human milk hormones. Additionally, participants used their own breast pumps for the milk collection rather than having a standardized breast pump. However, the potential influence of using different breast pumps was likely minimized because an investigator was present to help assure the breast had been emptied during collection. Given that a limitation of BIA is that it is influenced by fluid shifts, we standardized our protocol as much as possible and chose the 1-month assessment time point to minimize the potential confounding effect of early postpartum rapid fluid losses (Lukaski et al., 2007). Our ability to give further insight into potential programming influences of milk insulin, leptin, and adiponectin was also limited by the lack of infant outcomes in our dataset.
Conclusions
We add further evidence that maternal adiposity may be an important predictor of human milk leptin and insulin. We need more studies with rigorous assessments of maternal adiposity and standard procedures for the collection, storage, and analysis of human milk hormones in order to better understand factors influencing the composition of human milk. Although it cannot be disputed that human milk has many important benefits, it is crucial for us to understand modifiable factors that influence human milk composition and their effect on infant programming. Ultimately, it will be important to investigate whether optimization of human milk composition can prevent aberrant infant growth and improve long-term health, and what role medical professionals (i.e., International Board Certified Lactation Consultants®, Registered Dietitians) may play in this evolving field of research.
Funding:
This research was supported by an award from the UAB Health Services Foundation General Endowment Fund, by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award T32HL105349 and by the National Institute of Diabetes and Digestive and Kidney Diseases under Award P30DK079626. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Our work was funded by an award from the UAB Health Services Foundation General Endowment Fund. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award T32HL105349 and by the National Institute of Diabetes and Digestive and Kidney Diseases under Award P30DK079626. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Aundrea Harrison and Pooja Nagaraj for support with study execution, as well as Heather Hunter and Nikki C. Bush of the Human Physiology Core Laboratory for the UAB Diabetes Research Center for performing serum and human milk assays.
Footnotes
Conflicts of Interest: None.
References
- Badillo-Suárez PA, Rodríguez-Cruz M, & Nieves-Morales X (2017). Impact of metabolic hormones secreted in human breast milk on nutritional programming in childhood obesity. Journal of Mammary Gland Biology and Neoplasia, 22(3), 171–191. 10.1007/s10911-017-9382-y [DOI] [PubMed] [Google Scholar]
- Barker DJ (1990). The fetal and infant origins of adult disease. British Medical Journal, 301, 1111. 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Aguayo-Mazzucato C, & Weir GC (2016). Dynamic development of the pancreas from birth to adulthood. Upsala Journal of Medical Sciences, 121(2), 155–158. 10.3109/03009734.2016.1154906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosy-Westphal A, Schautz B, Later W, Kehayias JJ, Gallagher D, & Müller MJ (2013). What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. European Journal of Clinical Nutrition, 67(S1), S14–S21. 10.1038/ejcn.2012.160 [DOI] [PubMed] [Google Scholar]
- Bronsky J, Karpisek M, Bronska E, Pechova M, Jancikova B, Kotolova H, Stejskal D, Prusa R, & Nevoral J (2006). Adiponectin, adipocyte fatty acid binding protein, and epidermal fatty acid binding protein: Proteins newly identified in human breast milk. Clinical Chemistry, 52(9), 1763–1770. 10.1373/clinchem.2005.063032 [DOI] [PubMed] [Google Scholar]
- Brunner S, Schmid D, Zang K, Much D, Knoeferl B, Kratzsch J, Amann-Gassner U, Bader BL, & Hauner H (2015). Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatric Obesity, 10(1), 67–73. 10.1111/j.2047-6310.2014.222.x [DOI] [PubMed] [Google Scholar]
- Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, & Casanueva FF (1997). Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. The Journal of Clinical Endocrinology & Metabolism, 82(12), 4270–4273. 10.1210/jcem.82.12.4590 [DOI] [PubMed] [Google Scholar]
- Catalano PM, & Shankar K (2017). Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. British Medical Journal, 356. 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2018). Breastfeeding Report Card: United States, 2018. https://www.cdc.gov/breastfeeding/pdf/2018breastfeedingreportcard.pdf
- Chan D, Goruk S, Becker AB, Subbarao P, Mandhane PJ, Turvey SE, Lefebvre D, Sears MR, Field CJ, & Azad MB (2018). Adiponectin, leptin and insulin in breast milk: Associations with maternal characteristics and infant body composition in the first year of life. International Journal of Obesity, 42, 36–43. 10.1038/ijo.2017.189 [DOI] [PubMed] [Google Scholar]
- Chen C, Xu X, & Yan Y (2018). Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE, 13(8), e0202183. 10.1371/journal.pone.0202183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhreldin AR (2018). Maternal and infantile adiponectin as marker for anthropometric parameters of lactating mothers and their breast-fed infants. Indian Journal of Endocrinology and Metabolism, 22(1), 16–22. 10.4103/ijem.ijem_249_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, & Buchner A (2007). G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Fields DA, & Demerath EW (2012). Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatric Obesity, 7(4), 304–312. 10.1111/j.2047-6310.2012.00059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DA, George B, Williams M, Whitaker K, Allison DB, Teague A, & Demerath EW (2017). Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatric Obesity, 12(S1), 78–85. 10.1111/ijpo.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DA, Schneider CR, & Pavela G (2016). A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity, 24(6), 1213–1221. 10.1002/oby.21519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gába A, & Přidalová M (2016). Diagnostic performance of body mass index to identify adiposity in women. European Journal of Clinical Nutrition, 70(8), 898–903. 10.1038/ejcn.2015.211 [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Study 2015 Obesity Collaborators. (2017). Health Effects of Overweight and Obesity in 195 Countries over 25 Years. New England Journal of Medicine, 377, 13–27. 10.1056/nejmoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens GH (2017). The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obesity Facts, 10(3), 207–215. 10.1159/000471488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JN, Dunn-Meynell AA, Hartman TG, & Levin BE (2006). Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 291(3), R768–R778. 10.1152/ajpregu.00138.2006 [DOI] [PubMed] [Google Scholar]
- Henquin J-C, & Nenquin M (2018). Immaturity of insulin secretion by pancreatic islets isolated from one human neonate. Journal of Diabetes Investigation, 9(2), 270–273. 10.1111/jdi.12701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, Pemu A, & Rankin J (2019). The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Medicine, 16(6), e1002817. 10.1371/journal.pmed.1002817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseknecht KL, McGuire MK, Portocarrero CP, McGuire MA, & Beerman K (1997). Leptin is present in human milk and is related to maternal plasma leptin concentration and adiposity. Biochemical and Biophysical Research Communications, 240(3), 742–747. 10.1006/bbrc.1997.7736 [DOI] [PubMed] [Google Scholar]
- Jovanovic-Peterson L, Fuhrmann K, Hedden K, Walker L, & Peterson CM (1989). Maternal milk and plasma glucose and insulin levels: Studies in normal and diabetic subjects. Journal of the American College of Nutrition, 8(2), 125–131. 10.1080/07315724.1989.10720287 [DOI] [PubMed] [Google Scholar]
- Kim MK, Reaven GM, Chen Y-D, Kim E, & Kim SH (2015). Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity, 23(12), 2430–2434. 10.1002/oby.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaski HC, Hall CB, & Siders WA (2007). Assessment of change in hydration in women during pregnancy and postpartum with bioelectrical impedance vectors. Nutrition, 23(7–8), 543–550. https://doi-org.ezproxy3.lhl.uab.edu/10.1016/j.nut.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, Soeda J, Fernandez-Twinn DS, Martin-Gronert MS, Ozanne SE, Sigala B, Novelli M, Poston L, & Taylor PD (2010). Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. Journal of Hepatology, 52(6), 913–920. 10.1016/j.jhep.2009.12.042 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Cardoso RC, & Puttabyatappa M (2016). Developmental programming, a pathway to disease. Endocrinology, 157(4), 1328–1340. 10.1210/en.2016-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, & Shulman GI (2018). Mechanisms of insulin action and insulin resistance. Physiological Reviews, 98(4), 2133–2223. 10.1152/physrev.00063.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Franke K, & Kohlhoff R (2002). Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care, 25(1), 16–22. 10.2337/diacare.25.1.16 [DOI] [PubMed] [Google Scholar]
- Quinn EA, Largado F, Borja JB, & Kuzawa CW (2015). Maternal characteristics associated with milk leptin content in a sample of Filipino women and associations with infant weight for age. Journal of Human Lactation, 31(2), 273–281. https://doi.org/10.1177%2F0890334414553247 [DOI] [PubMed] [Google Scholar]
- Reifsnyder PC, Churchill G, & Leiter EH (2000). Maternal environment and genotype interact to establish diabesity in mice. Genome Research, 10(10), 1568–1578. 10.1101/gr.147000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodekamp E, Harder T, Kohlhoff R, Franke K, Dudenhausen JW, & Plagemann A (2005). Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: Role of the late neonatal period and early infancy. Diabetes Care, 28(6), 1457–1462. 10.2337/diacare.28.6.1457 [DOI] [PubMed] [Google Scholar]
- Sadr Dadres G, Whitaker KM, Haapala JL, Foster L, Smith KD, Teague AM, Jacobs DR Jr., Kharbanda EO, McGovern PM, Schoenfuss TC, Le LJ, Harnack L, Fields DA, & Demerath EW (2019). Relationship of maternal weight status before, during, and after pregnancy with breast milk hormone concentrations. Obesity, 27(4), 621–628. 10.1002/oby.22409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino F, Lupica MM, Benetti S, Petrucci E, Liguori SA, & Cordero Di Montezemolo L (2012). Adiponectin in breast milk: Relation to serum adiponectin concentration in lactating mothers and their infants. Acta Paediatrica, 101(10), 1058–1062. 10.1111/j.1651-2227.2012.02744.x [DOI] [PubMed] [Google Scholar]
- Scheja L, & Heeren J (2019). The endocrine function of adipose tissues in health and cardiometabolic disease. Nature Reviews Endocrinology, 15(9), 507–524. 10.1038/s41574-019-0230-6 [DOI] [PubMed] [Google Scholar]
- Shehadeh N, Khaesh-Goldberg E, Shamir R, Perlman R, Sujov P, Tamir A, & Makhoul IR (2003). Insulin in human milk: Postpartum changes and effect of gestational age. Archives of Disease in Childhood- Fetal and Neonatal Edition, 88(3), F214–F216. 10.1136/fn.88.3.F214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. (n.d.). Quick facts: Jefferson County, Alabama. U.S. Department of Commerce. Retrieved August 12, 2020, from https://www.census.gov/quickfacts/jeffersoncountyalabama [Google Scholar]
- Weyermann M, Beermann C, Brenner H, & Rothenbacher D (2006). Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clinical Chemistry, 52(11), 2095–2102. 10.1373/clinchem.2006.071019 [DOI] [PubMed] [Google Scholar]
- Weyermann M, Brenner H, & Rothenbacher D (2007). Adipokines in human milk and risk of overweight in early childhood: A prospective cohort study. Epidemiology, 18(6), 722–729. 10.1097/EDE.0b013e3181567ed4 [DOI] [PubMed] [Google Scholar]
- Whitmore TJ, Trengove NJ, Graham DF, & Hartmann PE (2012). Analysis of insulin in human breast milk in mothers with type 1 and type 2 diabetes mellitus. International Journal of Endocrinology. 10.1155/2012/296368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo BC, Barry VG, Ellis AC, & Gower BA (2018). Comparison of segmental body composition estimated by bioelectrical impedance analysis and dual-energy x-ray absorptiometry. Clinical Nutrition ESPEN, 28, 141–147. 10.1016/j.clnesp.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, & Taveras EM (2016). Risk factors for childhood obesity in the first 1,000 days: A systematic review. American Journal of Preventive Medicine, 50(6), 761–779. 10.1016/j.amepre.2015.11.012 [DOI] [PubMed] [Google Scholar]
- Young BE, Patinkin Z, Palmer C, de la Houssaye B, Barbour LA, Hernandez T, Friedman JE, & Krebs NF (2017). Human milk insulin is related to maternal plasma insulin and BMI: But other components of human milk do not differ by BMI. European Journal of Clinical Nutrition, 71(9), 1094–1100. 10.1038/ejcn.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Rong SS, Sun X, Ding G, Wan W, Zou L, Wu S, Li M, & Wang D (2018). Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: A longitudinal study. British Journal of Nutrition, 120(12), 1380–1387. 10.1017/s0007114518002933 [DOI] [PubMed] [Google Scholar]
- Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, & Campbell K (2018). Rapid weight gain during infancy and subsequent adiposity: A systematic review and meta-analysis of evidence. Obesity Reviews, 19(3), 321–332. 10.1111/obr.12632 [DOI] [PMC free article] [PubMed] [Google Scholar]


