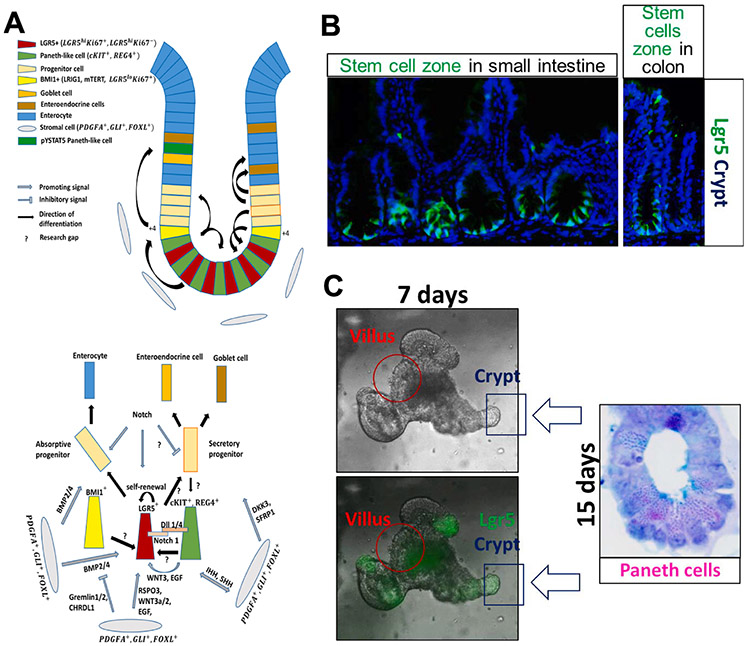
Fig. 4. Homeostasis in the intestinal crypt is maintained by IESC self-renewal and differentiation.
A) LGR5+ cells in the intestinal crypt proliferate to give rise to all other cell types of the intestinal epithelial monolayer, either via absorptive or secretory progenitor. There are 4–6 LGR5+ cells (red cells) in each crypt residing between cKIT+, REG4+ cells (green cells). All cells from the microenvironment contribute to the homeostasis by producing signals that promote either differentiation or self-renewal of LGR5+ stem cells. RSPO3, WNT3, EGF, and Notch support LGR5+ self-renewal, while BMP2/4, DKK3, and SFRP1 support differentiation into progenitor cells. The differentiation is tightly regulated by Gremlin1/2 and CHRDL1, as their increased expression at the base of the crypt inhibits BMP2/4, maintaining LGR5+ lineage at the base, but allowing BMP2/4 to promote enterocyte differentiation in the upper crypt area. Notch signaling is required for absorptive lineage differentiation, while “Notch off/WNT on” determines secretory lineages. Differentiating cells migrate upwards the crypt (black arrows showing the direction of differentiation), facing apoptosis at the top of the villus (not shown). However, fully differentiated, post-mitotic cells, cKIT+ REG4+, migrate towards the base of the crypt. cKIT+ REG4+ double-positive cells together with BMI1+ cells, residing at the +4 position, proliferate to maintain the LGR5+ stem cell pool. Research gaps in the key mechanism, which, in our opinion, could be the focus of future research and should investigate the possible cross talk or interference with STAT5 signaling, are marked by “?”. B and C) Representative images showing mouse intestinal epithelial stem cells and their epithelial niche cells in the in vivo crypt-villus (B) and in vitro organoids (C).