Table 1.
Reaction efficiencies and branching ratios for primary products
 |
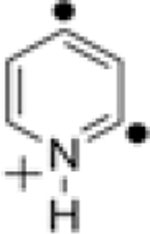 |
 |
 |
 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4/14 | 5 | ||||||
| S–T splittinga (kcal mol−1) |
−11.1 | −24.3 | −21.6 | −4.9b | −58.0 | |||||
| Relative energiesa (kcal mol−1) |
0 | −15.7 | −11.6 | 4.7/−22.8 | −35.8 | |||||
 |
H+ trns (2°) add | 78% | Hydride abs | 36% | 2 × H abs | 72% | H+ trns | 100% | H2O abs | 48% |
| H2O abs | 10% | CH2O abs | 26% | C2H4 abs | 20% | CH2O abs | 27% | |||
| Hydride abs | 5% | C2H4O abs | 20% | C3H3 abs | 8% | Hydride abs | 22% | |||
| CH2O abs | 4% | H2O abs | 14% | 2 × H abs | 2% | |||||
| 2 × H abs | 3% | 2 × H abs | 4% | C2H4O abs | 1% | |||||
| Efficiency = 85% | Efficiency = 28% | Efficiency = 1% | Efficiency = 99% | Efficiency = 84% | ||||||
 |
2 × H abs | 64% | 2 × H abs | 100% | 2 × H abs | 100% | No Reaction | Hydride abs | 52% | |
| Hydride abs | 22% | 2 × H abs | 48% | |||||||
| C2H4 abs | 9% | |||||||||
| C3H6 abs | 5% | |||||||||
| Efficiency = 39% | Efficiency = 0.03% | Efficiency = 0.1% | Efficiency = 70% | |||||||
| Less reactive isomer: 62% | Less reactive isomer: 23% | |||||||||
| Efficiency too low to be measured accurately | Efficiency = 8% | |||||||||
 |
H+ trns | 54% | Allyl abs | 49% | I abs | 69% | H+ trns | 100% | Iodide abs | 54% |
| I abs | (2°) I abs | (2°) I abs | ||||||||
| (2°) I abs | (2°) Allyl abs | (2°) Allyl abs | ||||||||
| (2°) Allyl abs | 36% | I abs | 42% | Allyl abs (2°) I abs | 29% | Allyl-H abs | 27% | |||
| (2°) I abs | (2°) Allyl abs | |||||||||
| (2°) Allyl abs | ||||||||||
| Allyl abs (2°) I abs | 6% | Allyl-H abs | 9% | Allyl-H abs | 2% | HI abs | 19% | |||
| Allyl-H abs | 4% | |||||||||
| Efficiency = 34% | Efficiency = 15% | Efficiency = 15% | Efficiency = 31% | Efficiency = 65% | ||||||
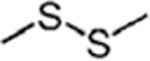 |
H+ trns | 65% | SCH3 abs | 73% | SCH3 abs | 82% | H+ trns | 100% | HSCH3 abs | 83% |
| (2°) SCH3 abs | (2°) SCH3 abs | |||||||||
| SCH3 abs | 17% | SSCH3 abs | 23% | SSCH3 abs | 12% | SCH2 abs | 14% | |||
| (2°) SCH3 abs | (2°) SCH3 abs | (2°) SCH3 abs | ||||||||
| e− trns | 8% | HSCH3 abs | 4% | HSCH3 abs | 3% | SCH3 abs | 3% | |||
| (2°) SCH3 abss | ||||||||||
| HSCH3 abs | 8% | SCH2 abs + CH3 abs | 3% | |||||||
| SSCH3 abs | 2% | |||||||||
| Efficiency = 93% | Efficiency = 47% | Efficiency = 58% | Efficiency = 100% | |||||||
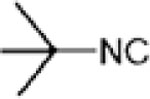 |
H+ trns | 48% | HCN abs | 53% | HCN abs | 79% | H+ trns and diss | 76% | Cyanide abs | 58% |
| (2°) C4H8 abs | (2°) C4H8 abs | |||||||||
| Cyanide abs | 28% | Cyanide abs | 47% | Cyanide absc | 21% | C4H8 abs | 24% | HCN abs | 42% | |
| (2°) C4H8 abs | ||||||||||
| C4H8 abs | 15% | |||||||||
| HCN abs | 9% | |||||||||
| (2°) C4H8 abs | ||||||||||
| Efficiency = 98% | Efficiency = 82% | Efficiency = 68% | Efficiency = 99% | Efficiency = 98% | ||||||
Reaction efficiencies are reported as kreaction/kcollision ×100.
abs, abstraction; trns, transfer; diss, dissociation; add, addition; LI, less reactive isomer; secondary products are noted as (2°) and are listed under the primary products that produce them.
Calculated at the RHF-UCCSD(T)/cc-pVTZ//UBPW91/cc-pVDZ level of theory and corrected for zero-point vibrational energy differences at 298 K by using the (unscaled) UBPW91/cc-pVDZ frequencies.
Calculated at the BD(T)/cc-pVTZ//UBPW91/cc-pVDZ level of theory; corrected for zero-point vibrational energy differences at 298 K by using the (unscaled) UBPW91/cc-pVDZ frequencies.
Note that proton transfer was erroneously reported[83] earlier.
BD(T), Brueckner; RHF, restricted Hartree–Fock; CCSD(T), coupled cluster with singles, doubles, and perturbative triples; cc-pVTZ, correlation-consistent polarized valence-triple-ζ; BPW91, gradient-corrected exchange functional of Becke combined with the gradient-corrected correlation functional of Perdew et al.; cc-pVDZ, correlation-consistent polarized valence-double-ζ.
