Fig. 1.
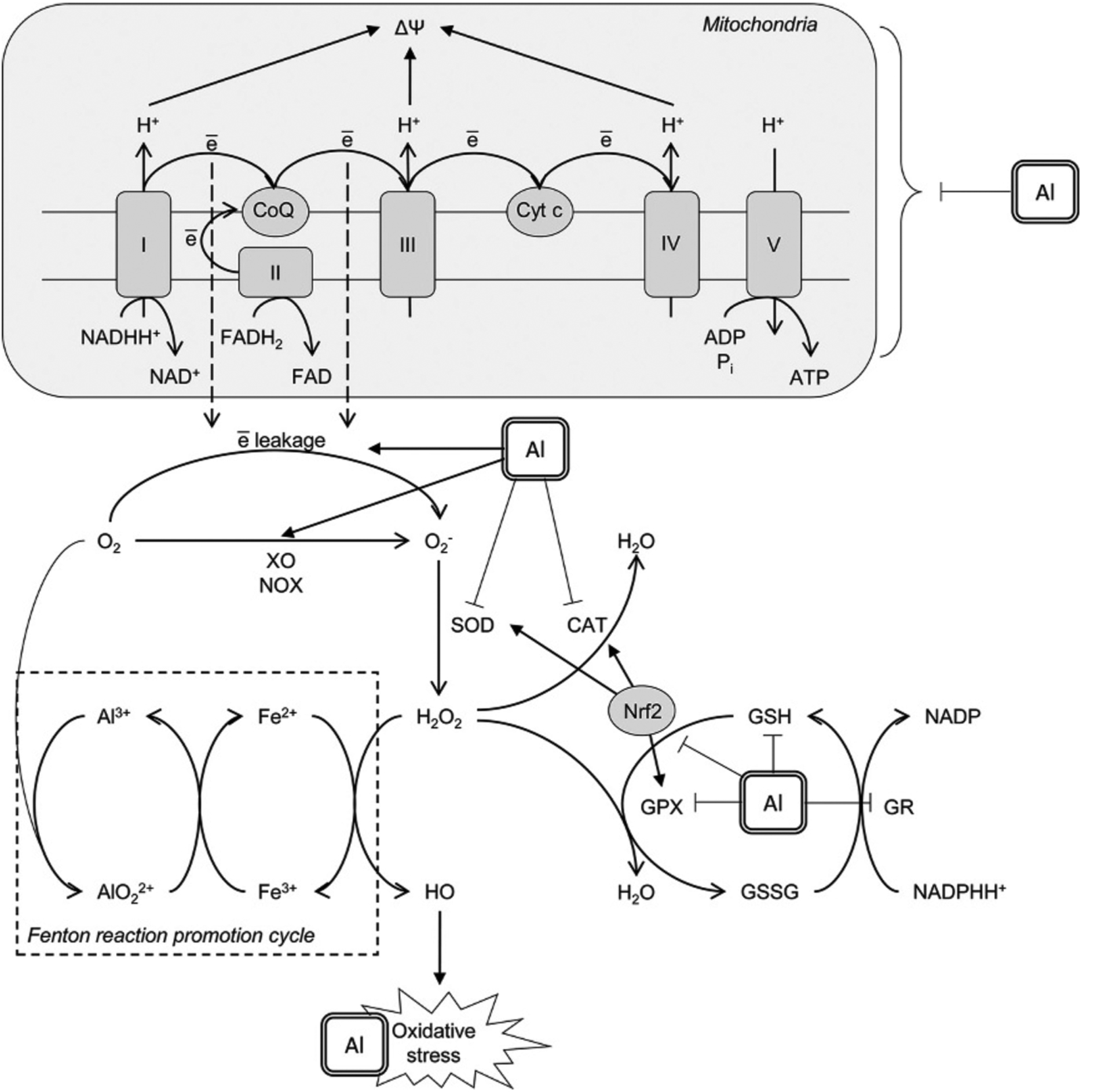
Mechanisms underlying prooxidant effect of aluminium (Al3+). Al3+ was shown to affect mitochondrial electron transport chain thus increasing electron leakage from Complex I and III with subsequent formation of superoxide anion radical . Another mechanism contributing to superoxide production involves Al-dependent increase in xanthine oxidase (XO) and NADPH-oxidase (NOX) activity. Al3+ cation is directly involved in the formation of highly reactive Al superoxide semi-reduced radical ion that was shown to promote prooxidant activity of Fe2+ in Fenton reaction with generation of hydroxyl radical (HO•). Prooxidant activity of Al is also aggravated by its inhibitory effect on enzymatic antioxidants including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR). The latter results in reduced glutathione (GSH) depletion. Moreover, Al was shown to down-regulate nuclear factor erythroid 2–related factor 2 (Nrf2), being the key regulator of the antioxidant system. Taken together, these mechanisms result in development of oxidative stress with increased oxidative modification of lipids, proteins and nucleic acids observed in brain/neuronal cell lines under Al exposure.
