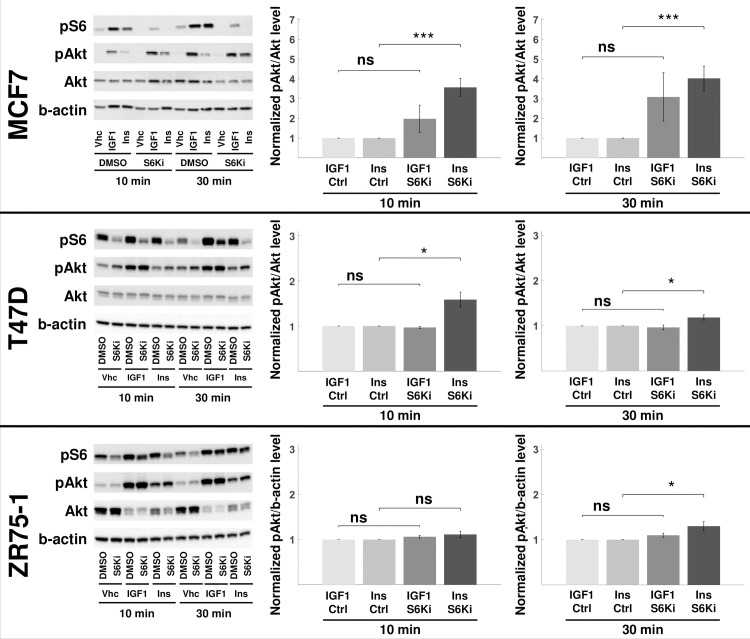
Fig 4. Ribosomal S6 kinase inhibition up-regulates Akt phosphorylation.
The RPS6K was inhibited in MCF7, T47D, and ZR75-1 cells. The pS6 levels are used as the proxy for S6K inhibition efficiency. The total Akt and b-actin levels are used to normalize pAkt and pS6 levels, respectively. The perturbagen, ligand, and time point for each sample are listed below the blot images (the rest of the replicates are shown in S9 Fig). The blot quantifications are shown on the right. The values are reported as normalized to the corresponding no-inhibition control (ctrl). pAkt levels represent the response of the cells to the perturbation. All MCF7, T47D, and ZR75-1 cell lines showed higher up-regulation of pAkt in insulin-stimulated cells at 30 min. The results are compared using an unpaired, one-tailed two-sample t-test, and P<0.05 (*), P<0.01 (**), P<0.005 (***), nonsignificant (ns). Results shown are mean ± s.e.m. of four independent replicates. S6 phosphorylation quantifications at 10 and 30 min are shown in S10 Fig.