Figure 16.
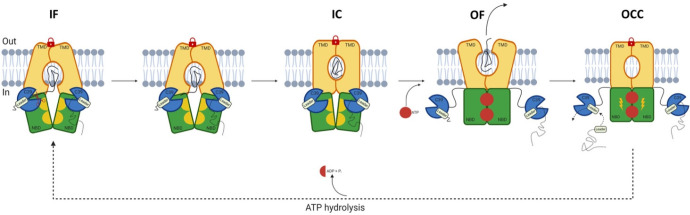
Modified alternating-access mechanism of PCAT1. CtA is recruited and cleaved in the inward-facing (IF) conformation. In the model by Kieuvongngam et al.,505 the substrate specificity of the transporter is conferred primarily, if not exclusively, by the interaction of the C39-domains with the leader peptide, and the transporter TMD essentially does not otherwise interact with the cargo. In a modification of the cycle proposed by Rachman and Mchaourab,522 the cargo is at the center of the interaction, as cargo interactions have been observed in transporter variants lacking C39-domains. In both cases, the interaction of the cargo proteins with the TMD leads to subsequent cleavage of the leader peptide, and a transient inward closed conformation (IC), similar to that observed in the McjD,459 is created, which enables the ATP binding. ATP-binding drives conformational changes in the TMD leading to occlusion of the cargo protein binding chamber, known as the outward occluded state (not shown). ATP binding stabilizes the outward-facing (OF) conformation in which the PEP domains are disengaged. After cargo release, TMDs isomerize to form an occluded state (OCC) in the absence of cargo. The energy of ATP-hydrolysis resets PCAT1 back to the inward-facing conformation, allowing PEP to dock into the TMD–NBD interface. Padlock indicates the closure of the outer “gate” of the transporter.