Figure 27.
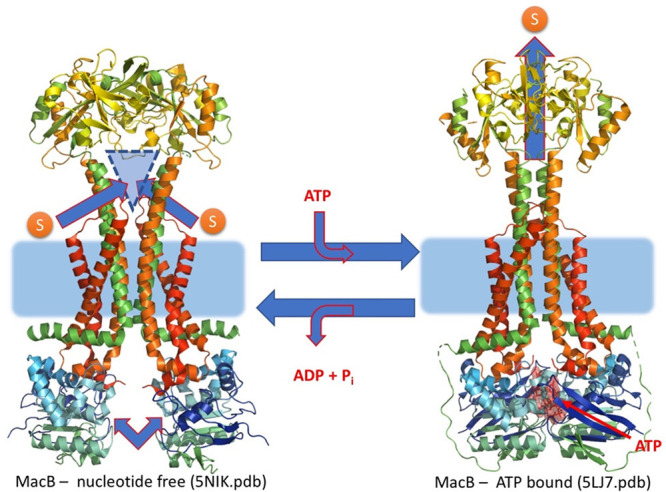
Mechanotransmission mechanism of MacB. Crystal structures suggest a possible cycling mechanism for MacB. In the nucleotide-free structures, the NDBs of the transporter are separated and appear unable to bind ATP. This coincides with the separation of the PCDs and lack of helical-bundle formation in the stalk helices. To the contrary, the ATP-bound forms show significant rearrangement of the PCDs, which also coincides with a helical bundle formation in the TM-domain, suggesting that the ATP-binding is communicated long-range from the NBDs across the membrane to the periplasmic domain through the TM-stalk helices. This has been dubbed “mechanotransmission”, as the stroke from ATP-binding is not used to transport the substrate across the membrane in which the ABC transporter resides but rather to cause conformational changes in the periplasm. (Modified, based on the model by Crow et al.431)
