Table 1. Summary of the Chemical Differences between Water- and Organic-Soluble Wood Tara.
| water-soluble | organic-soluble | |
|---|---|---|
| HR-Tof-AMS measurements | notable aldehyde (typical fragment of C2H3O+), carboxyl/peroxide (characteristic ion of CO2+), and methoxy (CH3O+), higher fraction of oxygenated fragments | high levels of aromatic hydrocarbon fragments, such as C2H2+, C3H3+, C6H5+, and C7H7+ |
higher O/C ratio and carbon
oxidation state (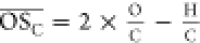 ) ) | ||
| FT-IR measurements | contain more −OH (3200–3600 cm–1), C=O (1630–1780 cm–1), and C–O (1040–1150 cm–1) structures | feature alkyl-alkenyl (2500–3100 cm–1) and aromatic C=C (1500–1700 cm–1 |
| REMPI mass spectra | contain PAHs, such as alkylated phenanthrenes including the softwood-combustion marker retene and pyrene phenols (e.g., phenol, methylphenols, methoxyphenols, etc.) had a higher relative abundance to the total peak intensity | contain PAH, such as alkylated phenanthrenes including the softwood-combustion marker retene, and furan-derivatives, such as (methylated) benzofurans and dibenzofurans relative abundance of PAH and their derivatives exceed relative abundance of phenols |
| GC×GC-HR-Tof-MS | O3 and O4 phenols, with functionalized substituents at the aromatic ring, such as coniferyl aldehyde (C10H10O3) or vanillic acid (C4H8O4), furans, and sugars | aromatic hydrocarbons (2.8% vs 0.4% semiquantitatively from peak intensity) but less furans (22.9% vs 32.3%) |
| O1/O2 phenols, total phenols (sum of O1- to O4-phenols) in the organic-soluble wood tar (41.8% vs 31.0%) |
The percentages are semiquantitative, as derived from signal intensities.
