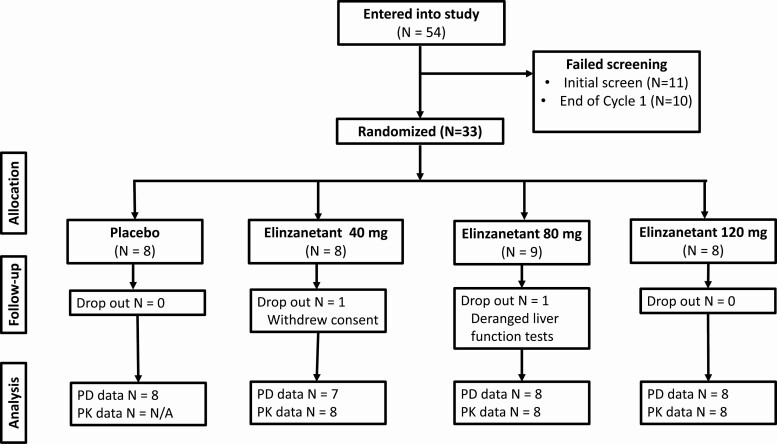
Figure 1.
Consolidated Standards of Reporting Trials participant flow depicts the number of participants entered into the study, randomly assigned, and analyzed. Fifty-four women were recruited and screened, of whom 33 were randomly assigned to receive placebo or elinzanetant 40 mg, 80 mg, or 120 mg (N = 8 or 9 per group). The most common reason for screen failure was menstrual period duration or irregularity. One woman in each of the elinzanetant 40-mg and 80-mg groups did not complete the treatment course. A total of 31 participants were included in the pharmacodynamic (PD) per-protocol analyses. Twenty-four participants from the elinzanetant 40-mg, 80-mg, and 120-mg groups were included in the pharmacokinetic (PK) analysis.