Figure 5.
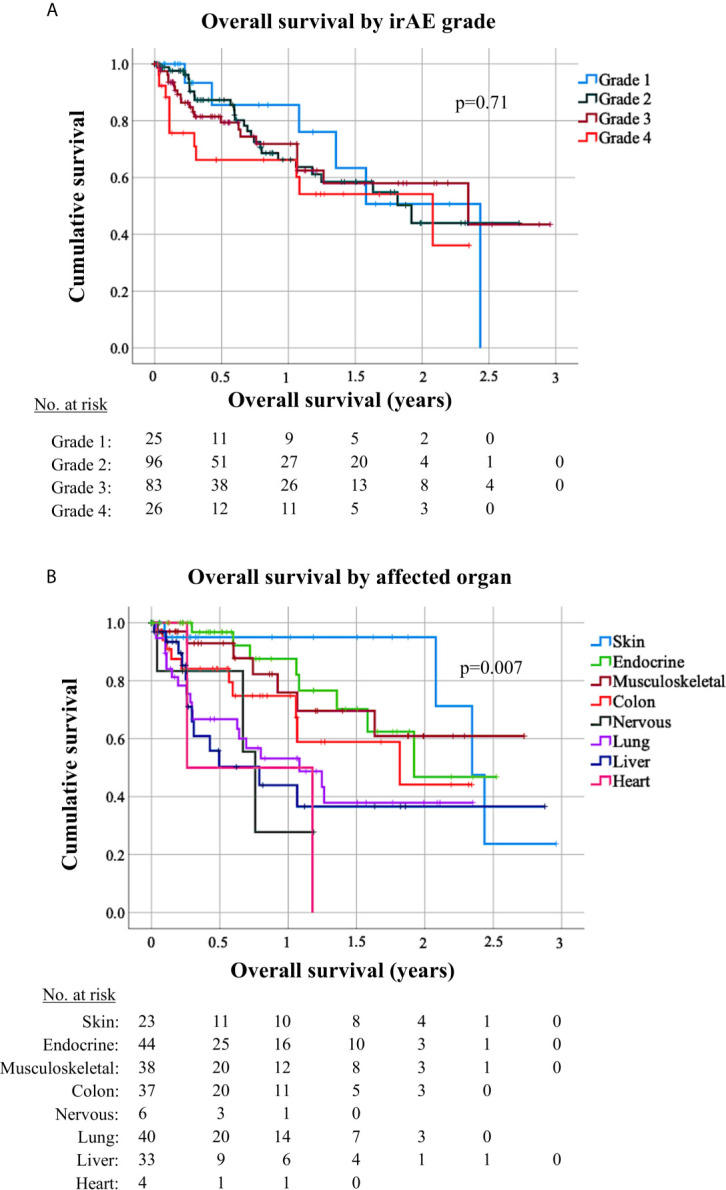
Survival of patients with grade 1–4 immune-related adverse events by affected organ. (A) Overall survival (OS) from start of immunotherapy for non-small-cell lung cancer (NSCLC) patients developing immune-related adverse events (irAEs) did not differ significantly by irAE grade (logrank p = 0.71). Median OS was 29 months [95% confidence interval (CI) n/a] in case of grade 1 irAE, 23 months (13.0–31.2) in case of grade 1 irAE, 28 months (3.7–52.6) in case of grade 3 irAE, and 25 months (8.5–41.4) in case of grade 4 irAE. (B) OS for NSCLC patients developing irAE showed significant differences according to the irAE type (logrank p = 0.007). Median OS was 28.1 months (CI 23.9–32.3) for patients with dermatologic irAE, with 2-year OS rate 95% (CI 85–100); 23 months (CI n/a) for patients with endocrinologic irAE, with 2-year OS rate 47% (CI 15–79); not reached for patients with musculoskeletal irAE, with a 2-year OS rate 61% (36–85); 22 months (3.1–40.6) for patients with colitis, with a 2-year OS rate 44% (14–75); 13 months (4.2–21.8) for patients with pneumonitis, with a 2-year OS rate 38% (19–57); and 9.5 months (1.4–17.6) for patients with hepatitis, with a 2-year OS rate 37% (14–59); 9.1 months (7.3–10.9) with a 2-year OS rate of 27.8% (CI 0–73) for patients with neurological irAE; and 3.1 months (CI na) for patients with cardiologic irAE with a 2-year OS rate 0%. Only irAE with >3 occurrences in our patients were included in this analysis.
