Figure 1.
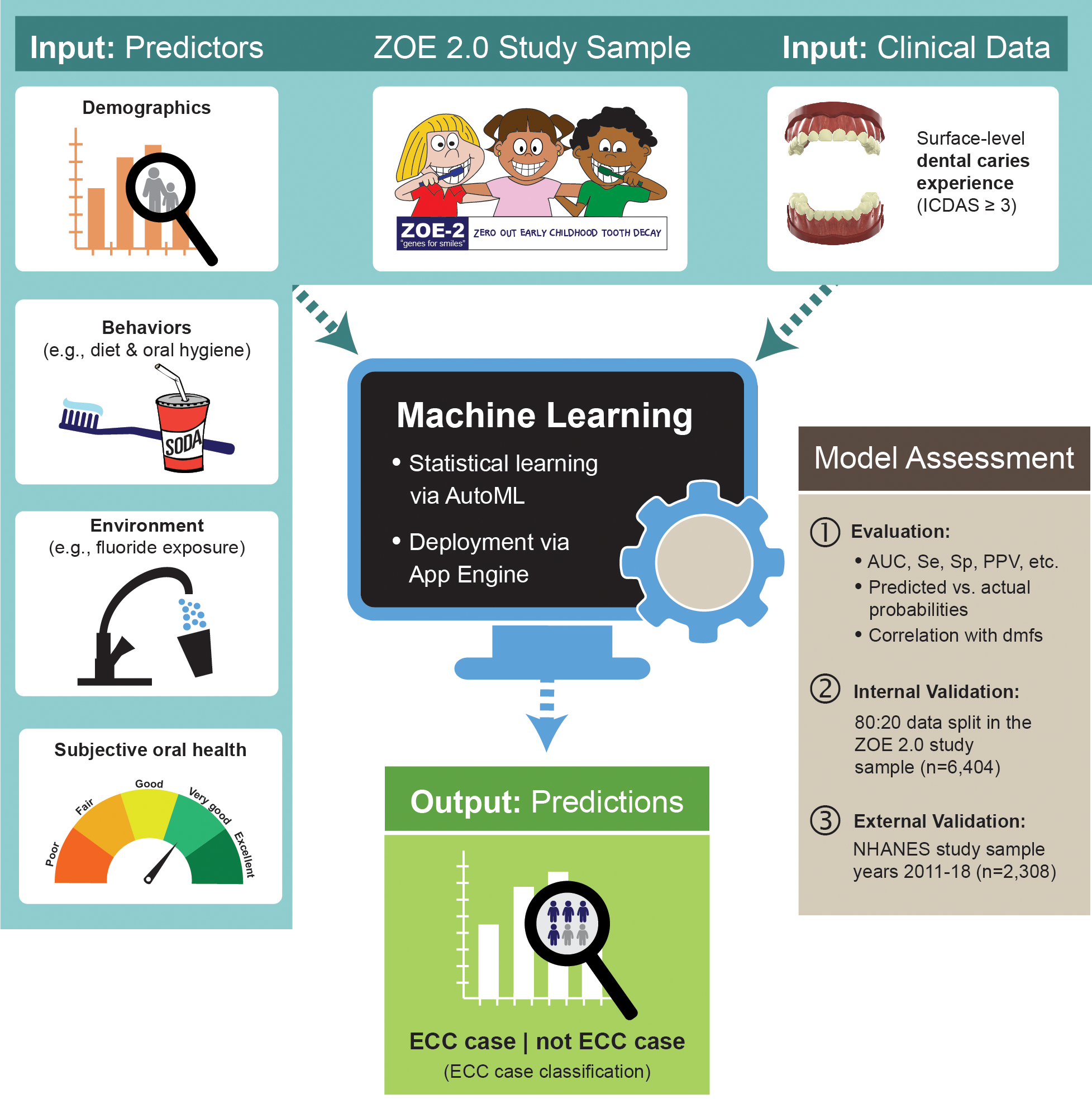
Overview of study data, model development, and assessment procedures. First, clinical ECC data and information on postulated ECC predictors from 6,404 three- to five-year-old participants of the ZOE 2.0 study were used to develop an ECC classification model. An automated machine learning (AutoML) deployment on Google Cloud was used to identify the best-performing model, as determined by conventional classification metrics such as the area under the receiver operator curve (AUC), sensitivity (Se), specificity (Sp), and positive predictive value (PPV). The model was internally validated using a customary 80:20 data split, wherein the model is developed in a random 80 percent of the sample and then evaluated in the remaining 20 percent. External replication of the best-performing model developed in the ZOE 2.0 study was done using nationally representative data from the National Health and Nutrition Examination Survey (NHANES) study years 2011 to 2018 (N equals 2,308, similarly aged children).
