To the Editor,
The outbreak of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), disproportionately affects certain populations, such as older individuals.1 In contrast, children comprise <2% of cases and generally exhibit milder symptoms.2 These disparate observations in children versus adults may be attributed to both environmental and host immune factors, including an immature and developing immune system.3 A key breakthrough in understanding the infectious mechanism of COVID-19 was the discovery of angiotensin-converting enzyme 2/ACE2, the receptor that SARS-CoV-2 uses to gain entry into human host cells.4 ACE2 expression is observed in the upper and lower respiratory tract and in extrapulmonary tissues that are prone to SARS-CoV-2 infection (e.g. gastrointestinal tract, cardiovascular system, skin).5 Lower maturity and function of ACE2 have been hypothesized to explain less disease prevalence in children.3
Recently, one study evaluated ACE2 gene expression in nasal epithelial samples in children and adults, reporting higher ACE2 expression in older children (10-17 years old) and adults (≥18 years old) versus younger children (<10 years old), and these associations remained significant after adjusting for sex and asthma.6 Radzikowska and Ding et al7 also investigated ACE2 expression and other COVID-19-related genes across various tissues from healthy subjects and individuals with comorbidities and risk factors suspected to predispose COVID-19, identifying distinct mRNA expression profiles in different demographic cohorts that vary by tissue type. Sward et al8 analyzed soluble ACE2 protein expression, which may capture COVID-19 disease risk and/or high disease activity, in the serum of various pediatric and young adult age-groups, showing differences between males and females beginning in adolescence. However, this study did not assess infants and toddlers and was limited by only following the subjects into young adulthood (mean age: 23.5).8 Therefore, ACE2 protein expression differences in the serum of very young patients (i.e. infants/toddlers) versus adults, as well as potential associations with atopic conditions (e.g. asthma or atopic dermatitis/AD), remain poorly understood.
To expand on prior investigations, we used Olink to evaluate the protein expression of ACE2 in the serum of a previously published cohort of 19 healthy infants and toddlers (age ≤5 years old, mean age: 2.1; 52.6% females) compared with 17 healthy adults (age range: 24-55, mean age: 41; 35.3% females; Table S1).9 To understand whether ACE2 protein expression is primarily associated with age, or can also be affected by coexisting atopic dermatitis/AD, a common inflammatory condition characterized by type 2 systemic inflammation in patients with moderate-to-severe disease (but in which increased mortality/morbidity from COVID-19 has not yet reported), we also evaluated ACE2 expression in previously reported infant/toddler and adult cohorts with moderate-to-severe AD.9 We included 29 infants and toddlers with moderate-to-severe AD (age ≤5 years old, mean age: 1.8 years, 48.3% females, SCORAD 51 ± 3 [mean ± SE]) and 55 adults with moderate-to-severe AD (age range: 18-72, mean age: 40, 47.3% females, SCORAD 54 ± 1.8 [mean ± SE]; Table S1). No healthy subjects or AD patients had a history of cardiovascular disease, hypertension, diabetes, or associated medications.9 None of the control adults and 17 of the AD adults had a history of mild asthma, controlled by inhalers. No pediatric AD patients or controls had asthma.
Blood was collected and centrifuged, and plasma was stored at −80°C. Aliquots were analyzed by the Proteomic Olink Proseek multiplex assay, as described.9 ACE2 and CTSL1 proteins from the CVD II panel were assessed. Additional details can be found in Appendix S1. Significant differences in protein expression profiles were determined by a two-tailed Student’s t-test, and correlation coefficients were computed using Spearman’s correlation.
We observed significantly greater ACE2 protein expression in the serum of healthy adults versus healthy infants and toddlers (P = 1.15e-06; Figure 1A). Similarly, higher ACE2 expression was seen in adults with AD versus infants and toddlers with AD (P = 7.53e-18; Figure 1A). No significant differences were seen in ACE2 protein expression between AD adults versus healthy adults (P = .6), nor between AD infants/toddlers versus healthy infants/toddlers (P = .44). Using Spearman’s correlation, we identified a positive correlation between ACE2 expression and age (r = 0.76, P = 2.9e-07; Figure 1B).
FIGURE 1.

A, Graph depicts mean ACE2 protein expression (colored circles), represented in normalized protein expression (NPX) values, in the serum of the healthy pediatric cohort, healthy adult cohort, AD pediatric cohort, and AD adult cohort. The limit of detection (LOD) for ACE2 protein is shown in the y-axis label. Horizontal bars denote standard error. P-values between adults versus pediatric cohorts are shown. B, Correlation scatterplot between ACE2 protein expression and age in healthy infants and toddlers and healthy adults. Light blue circles denote cohort of infants and toddlers, and dark blue circles denote cohort of adults. Gray shaded area denotes the 95% confidence interval. Spearman’s correlation coefficient and P-value are provided. C, Graph depicts mean ACE2 protein expression (colored circles), represented in normalized protein expression (NPX) values, in the serum of healthy and AD pediatric and adult cohorts by gender. The limit of detection (LOD) for ACE2 protein is shown in the y-axis label. Horizontal bars denote standard error. P-values between various groups are shown. AD, atopic dermatitis; ACE2, angiotensin-converting enzyme 2; NPX, normalized protein expression; LOD, limit of detection
Gender analysis revealed significantly greater ACE2 expression in healthy adult females versus healthy pediatric females, as well as healthy adult males versus healthy pediatric males (P < .05; Figure 1C). Furthermore, adult males showed significantly greater ACE2 protein expression in the serum versus adult females, regardless of AD (P < .05; Figure 1C). However, there were no significant male-female differences among healthy or AD children.
Cathepsin L/CTSL1 is a protease that has been shown to cleave and prime the SARS-CoV-1 spike protein,10 and as such, this enzyme has been hypothesized to similarly play a role in SARS-CoV-2 infection.4 We therefore investigated the protein expression of CTSL1 in both healthy and AD cohorts and observed that adults have significantly greater CTSL1 protein expression in the serum versus infants and toddlers (P < .001; Figure 2A). In addition, ACE2 protein expression and CTSL1 protein expression are significantly and positively correlated (r = 0.75 P = 8.52e-07; Figure 2B).
FIGURE 2.
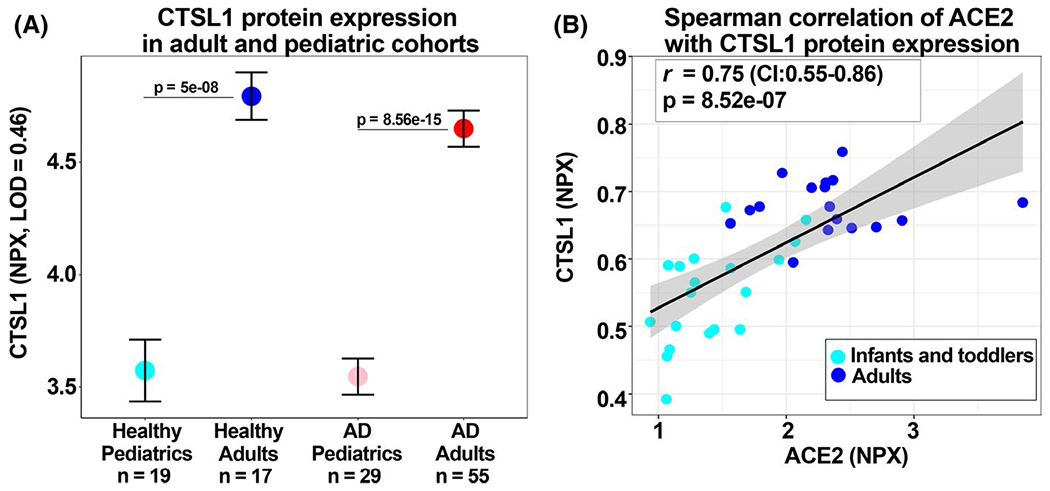
A, Graph depicts mean CTSL1 expression (colored circles), represented in normalized protein expression (NPX) values, in the serum of the healthy pediatric cohort, healthy adult cohort, AD pediatric cohort, and AD adult cohort. The limit of detection (LOD) for CTSL1 protein is shown in the y-axis label. Horizontal bars denote standard error. P-values between adults versus pediatric cohorts are shown. B, Correlation scatterplot between ACE2 protein expression and CTSL1 protein expression in healthy infants and toddlers and healthy adults. Light blue circles denote cohort of infants and toddlers, and dark blue circles denote cohort of adults. Gray shaded area denotes the 95% confidence interval. Spearman’s correlation coefficient and P-value are provided. AD, atopic dermatitis; CTSL1, cathepsin 1; NPX, normalized protein expression; LOD, limit of detection
A sensitivity analysis of adult AD patients with and without concomitant asthma also showed similar differences versus the pediatric age-group (P < .001), with no significant differences between adult AD patients with and without asthma (P = .79, Figure S1A). We further evaluated additional clinical parameters, where available, and found no correlation of ACE2 protein expression with the AD severity measure, SCORAD (Figure S1B), nor with pruritus or serum IgE in AD patients, or body mass index/BMI in healthy or AD cohorts (Table S2).
A sensitivity analysis by race/ethnicity also maintained significance between age-groups. A trend toward significantly greater ACE2 expression was observed in healthy African American adults versus healthy European American adults, as well as in European American AD infants/toddlers versus both Asian and African American AD infants/toddlers (P < .1; Figure S1C).
To our knowledge, we demonstrate for the first time that adults have significantly greater ACE2 protein expression in serum compared with infants and toddlers. When examining differences by sex within both adult cohorts, ACE2 expression was consistently higher in males versus females, while no differences between sexes were observed in the pediatric cohort. Further, we obtained a robust and significant positive correlation between age and ACE2 protein expression. Our results complement previous transcriptomic reports in skin and serum7,8 with novel proteomic data that mirror the clinical observations of generally higher disease prevalence and severity of SARS-CoV-2 infections in older adult populations versus younger children and higher mortality risk associated with the male sex.1 Additionally, our data also demonstrate significantly higher CTSL1 serum protein expression in adults versus infants/toddlers with and without AD, as well as a significant, positive correlation between ACE2 protein expression and CTSL1 serum protein expression. Finally, our data extend the recent data from asthma,6 further suggesting that atopy does not pose an increased risk of COVID-19 morbidity.
In sum, while additional studies are needed to uncover the mechanistic basis of varying COVID-19 clinical presentations, our data associate significantly higher ACE2 protein expression in the serum of adults compared with infants and toddlers, and in adult males compared with adult females. These data suggest the potential systemic role of ACE2 protein levels in the differential clinical manifestations among various patient populations.
Supplementary Material
Abbreviations:
- ACE2
angiotensin-converting enzyme 2
- AD
atopic dermatitis
- BMI
body mass index
- CDC
centers for disease control and prevention
- COVID-19
coronavirus disease 2019
- CTSL1
cathepsin L
- EASI
eczema activity and severity index
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SCORAD
SCORing atopic dermatitis
- SE
standard error
Footnotes
Pavel, Wu and Renert-Yuval joint first-authorship
CONFLICTS OF INTEREST
Dr Pavel, Ms Wu, Dr Renert-Yuval, Dr Del Duca, and Dr Miller have nothing to disclose. Dr Paller reports grants from AbbVie, AnaptysBio, Celgene, Eli Lilly, Galderma, Incyte, Leo, Janssen, Novartis, and Regeneron, during the conduct of the study; and other from Almirall, Amgen, Asana, Boehringer Ingelheim, Castle Creek, Celgene, Dermavant, Dermira, Eli Lilly, Exicure, Forte, Galderma, Lenus, Leo, MEDACorp, Meiji Seika, Novan, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sol-Gel, and UCB, outside the submitted work. Dr Krueger reports grants and personal fees from Pfizer, Amgen, Janssen, Lilly, Merck, Novartis, Kadmon, Dermira, Boehringer, Innovaderm, Kyowa, BMS, Serono, Biogen Idec, Delenex, AbbVie, Sanofi, Baxter, Parexel, Xenoport, and Kineta, during the conduct of the study. Dr Guttman-Yassky reports grants from AbbVie, Celgene, Eli Lilly, Janssen, MedImmune/Astra Zeneca, Novartis, Pfizer, Regeneron, Vitae, Glenmark, Galderma, Asana, Innovaderm, Dermira, and UCB, during the conduct of the study; and other from Sanofi Aventis, Regeneron, Stiefel/GlaxoSmithKline, MedImmune, Celgene, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae, LEO Pharma, AbbVie, Eli Lilly, Kyowa, Mitsubishi Tanabe, Asana Biosciences, and Promius, outside the submitted work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus disease 2019 in children — United States, february 12-april 2, 2020. MMWR Morb Mortal Wkly Rep. 2019;2020(69):422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6). [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26 and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75:2828–2844. 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swärd P, Edsfeldt A, Reepalu A, Jehpsson L, Rosengren BE, Karlsson MK. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit Care. 2020;24(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner PM, He H, Pavel AB, et al. The blood proteomic signature of early-onset pediatric atopic dermatitis shows systemic inflammation and is distinct from adult long-standing disease. J Am Acad Dermatol. 2019;81(2):510–519. [DOI] [PubMed] [Google Scholar]
- 10.Bosch BJ, Bartelink W, Rottier PJM, Cathepsin L. Functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol. 2008;82(17):8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


