Figure 4.
Cryo-EM of human and yeast R2TP-TTT complexes
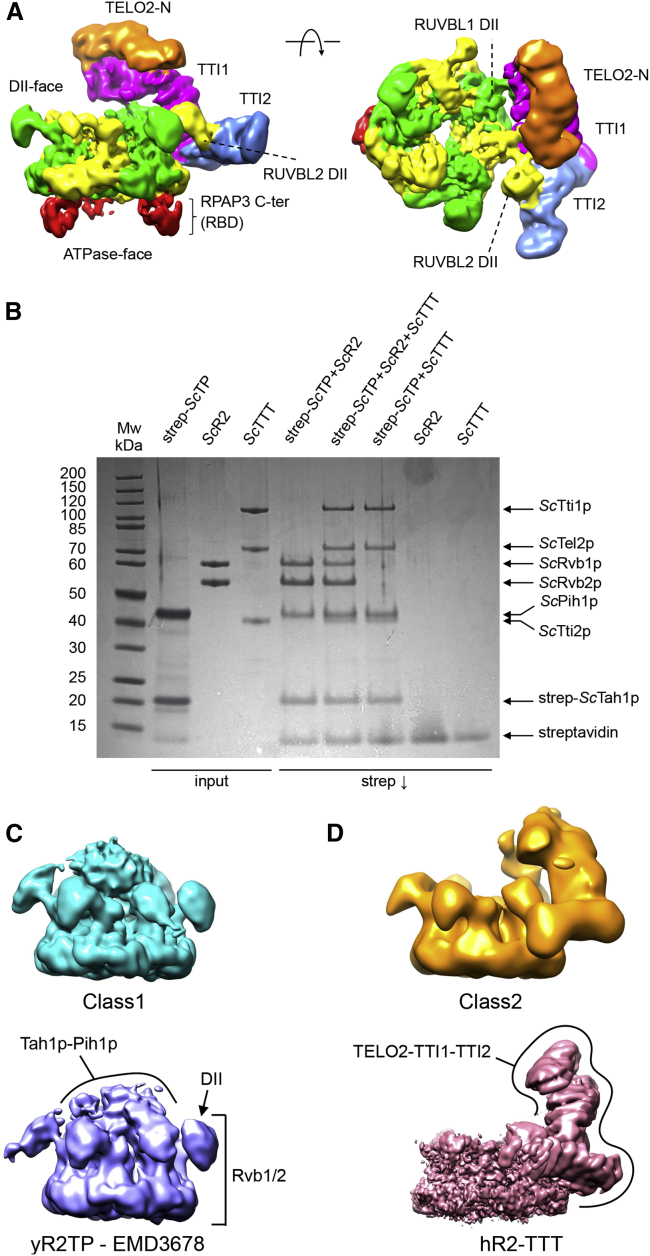
(A) Two views of human R2TP-TTT volume: RUVBL1-RUVBL2 hexamer, green and yellow respectively; TTI1, magenta; TTI2, blue; TELO2-N, orange. The RPAP3 C-terminal RUVBL2-binding domain (RBD) is identifiable in the ATPase face of each of the RUVBL2 subunits (red).
(B) Coomassie-stained SDS-PAGE gel showing analysis of interaction of yeast Tah1p-Pih1p (yTP), Rvb1p-Rvb2p (yR2), and Tel2p-Tti1p-Tti2p (yTTT) subcomplexes. Pull-down on the tandem strep-tag attached to the N terminus of Tah1p within the yTP complex co-precipitates yR2 and yTTT simultaneously and as separate co-complexes.
(C) Cryo-EM analysis of co-precipitated Tah1p-Pih1p-Rvb1p-Rvb2p-Tel2p-Tti1p-Tti2p (yeast R2TP-TTT). 3D classification yields two main classes: one class contained particles with strong central density between the DII insertion domains of the R2 ring comprising two-thirds of the particles (top), and the other class resembled the previously described yeast R2TP complex (EMD-3678) (Rivera-Calzada et al., 2017) (bottom).
(D) As in (C), but showing the second main class comprising two-thirds of the particles, which lack the central density attributed to Tah1p-Pih1p, but display strong peripheral density at one edge of the R2 ring (top) that strongly resembles the density for the TTT complex in the higher resolution human R2TTT structure (bottom). No significant class of particles was identified in which the central and peripheral density features were simultaneously present.