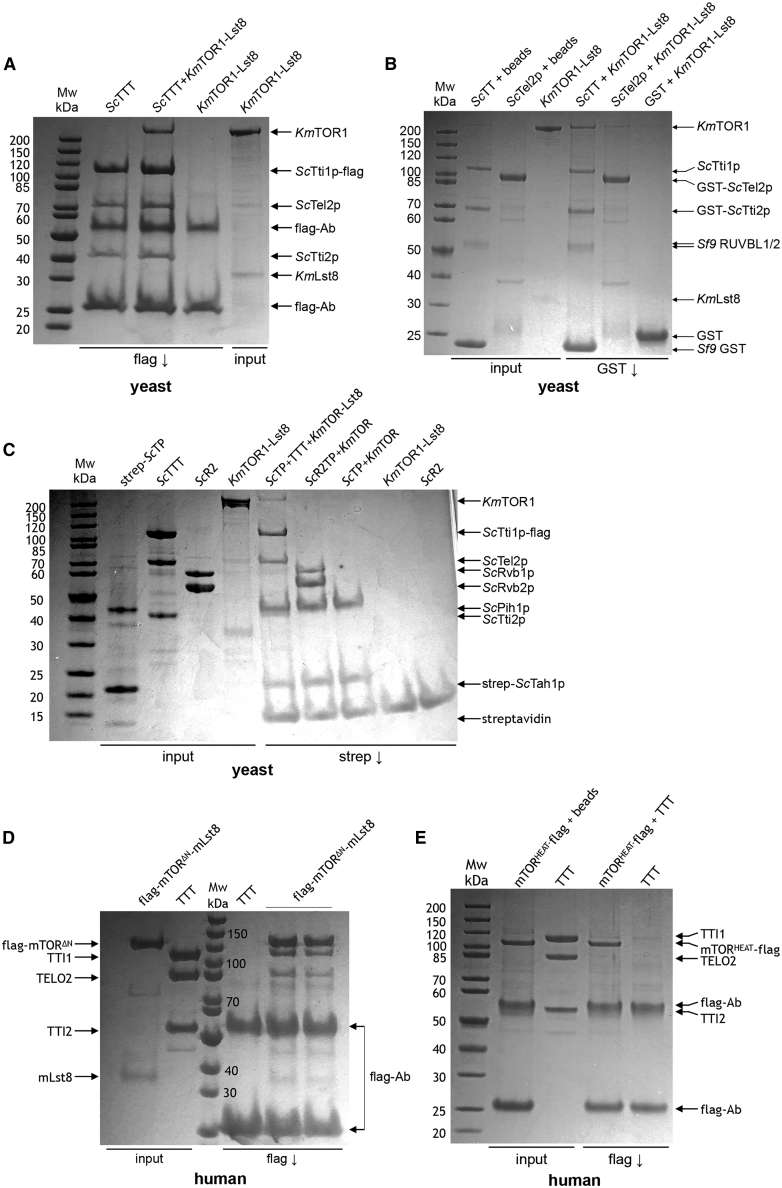
Figure 5.
Mapping the interactions between TTT and TOR
(A) Coomassie-stained SDS-PAGE gel showing interaction of yeast (Kluyveromyces marxianus) TOR1-Lst8 with yeast (S. cerevisiae) TTT. FLAG-tagged Tti1p co-immunoprecipitates TOR1, whereas anti-FLAG beads alone do not. Lst8, which is evident as a weakly staining band in the input, was not evident in the immunoprecipitated TTT-TOR1.
(B) As in (A), but for interaction of KmTOR-Lst8 with GST-tagged Tti2p-Tti1p or GST-tagged Tel2p. KmTOR was co-precipitated in GST pull-down with Tti1p-GST-TTI2p, but not with Tel2p.
(C) As in (A), but for interaction of KmTOR-Lst8 with TP and R2TP complexes containing strep-tagged Tah1p. Neither TP nor R2TP complexes were able to co-precipitate KmTOR directly. However, tagged TP could co-precipitate KmTOR when TTT was present, confirming that TP and KmTOR do not compete for binding to TTT.
(D) As in (A), but for interaction of a FLAG-tagged N-terminally truncated human mTOR construct (mTORΔN) co-expressed with mLST8, with human TTT. TTT is co-immunoprecipitated by FLAG-tagged mTORΔN, but not by FLAG beads alone.
(E) As in (A), but for interaction of a FLAG-tagged soluble N-terminal fragment of human mTOR encompassing the major segment of HEAT repeats (mTORHEAT) with human TTT. Flag-tagged mTORHEAT was precipitated by FLAG beads, but it did not co-immunoprecipitate TTT.