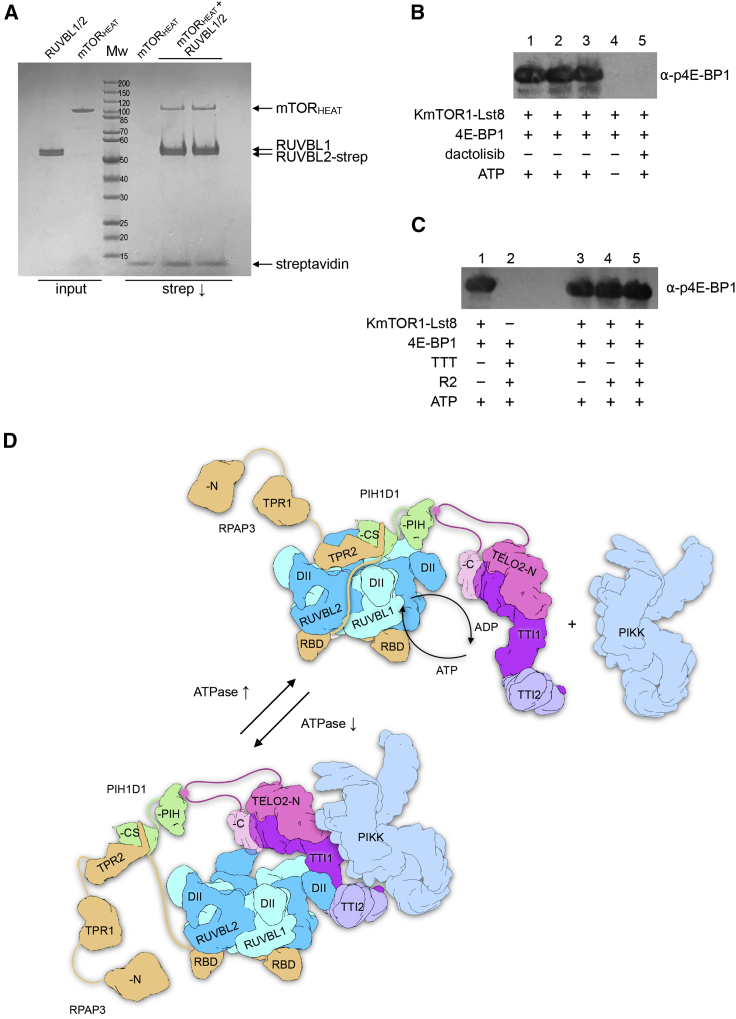
Figure 6.
TTT couples mTOR recruitment to R2TP to regulation of the chaperone
(A) Coomassie-stained SDS-PAGE gel showing the interaction of an mTOR fragment comprising the N-terminal HEAT region (residues 1–929) with the strep-tagged RUVBL1-RUVBL2 complex.
(B) Western blot showing phosphorylation of 4E-BP1 by the yeast (K. marxianus) TOR1-Lst8 complex. A strong signal was detected by a phosphospecific antibody to pThr37/46 in the presence of ATP, but not when ATP was omitted from the reaction, or when the ATP-competitive mTOR inhibitor dactolisib (NVP-BEZ235) was present.
(C) As in (B), but with the addition of TTT, R2, or both. Neither of these chaperone components, alone or in combination, affects its ability to phosphorylate a substrate.
(D) Model of the functions of TTT. Flexible tethering to the R2 ring allows for facile exchange of TP and TTT components at the main interaction site on the DII domain face of the ring. Interaction of the PIH1D1 part of TP with a RUVBL1 DII domain facilitates partial ring opening and nucleotide exchange (Munoz-Hernandez et al., 2019), accelerating the basal ATPase activity of the ring (Rivera-Calzada et al., 2017). The CK2 phosphorylation site within the inherently disordered linker connecting the N- and C-terminal domains of TELO2 (Horejsí et al., 2010) tethers the TTT subcomplex to the R2 ring through interaction with the PIH domain of PIH1D1 (Hořejší et al., 2014; Pal et al., 2014) and thereby facilitates recruitment of a PIKK client to the core complex (top). Rearrangement of the complex allows TTT to bind to the DII domains of consecutive RUVBL1-RUVBL2 domains, fixing them in an ADP-bound closed state that downregulates the basal ATPase, potentially bringing the PIKK client into closer association. Steric hindrance of the DII domains, as well as allosteric incompatibility, prevents simultaneous binding of PIH1D1 to the DII face of the ring. Although not engaged, TP remains tethered to the complex through the interaction of the PIH domain with the TELO2 linker segment (see above) and the interaction of the C-terminal domain of RPAP3 with the ATPase face of the R2 ring (bottom).