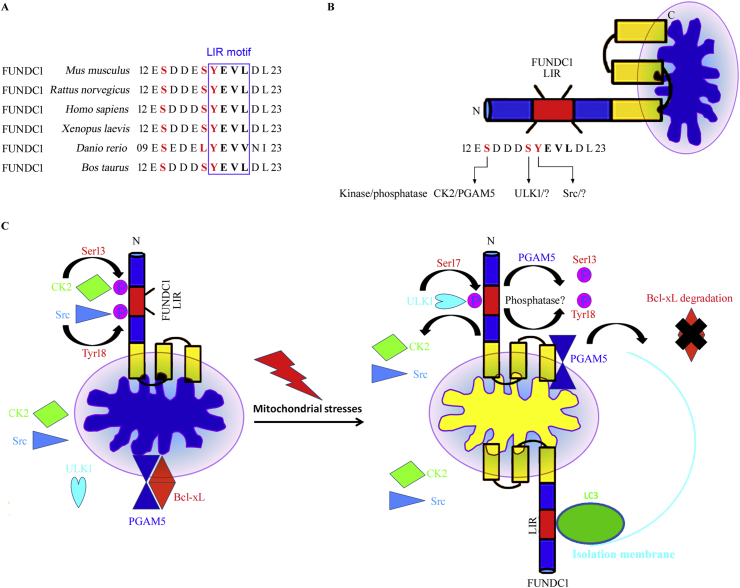
Figure 3.
Regulatory mechanism of mitochondrial stresses (hypoxia, et al)-induced FUNDC1-dependent mitophagy in mammalian systems. (A) Conserved LIR domain sequences between different species are demonstrated. (B) Schematic representation of the mitochondrial location, structure and reversible phosphorylation of FUNDC1 at the illustrated critical amino acide residues in the mitochondrial outer membrane region. (C) Under physiological conditions, the protein kinases of casein kinase 2 (CK2) and Src phosphorylate FUNDC1 at Ser13 and Tyr18, respectively, therefore suppressing the interaction between FUNDC1 and LC3 and FUNDC1-mediated mitophagy. BCL-XL interacts with and blocks PGAM5 under normoxia conditions. Under mitochondrial stress conditions, such as hypoxia treatment or uncoupler carbonylcyanide p-trifluoromethoxy-phenylhydrazone (FCCP) stimulation, CK2 and Src cannot phosphorylate FUNDC1 at Ser13 and Tyr18, whereas phosphoglycerate mutase family member 5 (PGAM5) is released from BCL-XL, promoting the dephosphorylation of FUNDC1 at Ser13 to enhance FUNDC1-mediated mitophagy. Simultaneously, ULK1 can interact with and phosphorylate FUNDC1 at Ser17, thus promoting the FUNDC1–LC3 interaction and the subsequent autophagic degradation of mitochondria. However, the phosphatases responsible for the dephosphorylation of FUNDC1 at Ser13 and Tyr18 remain to be explored.