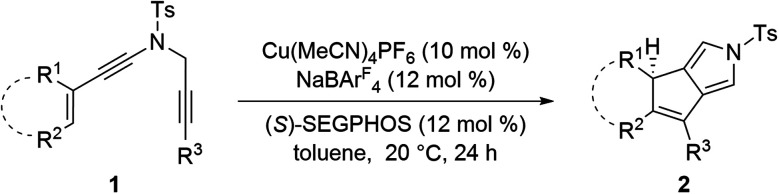
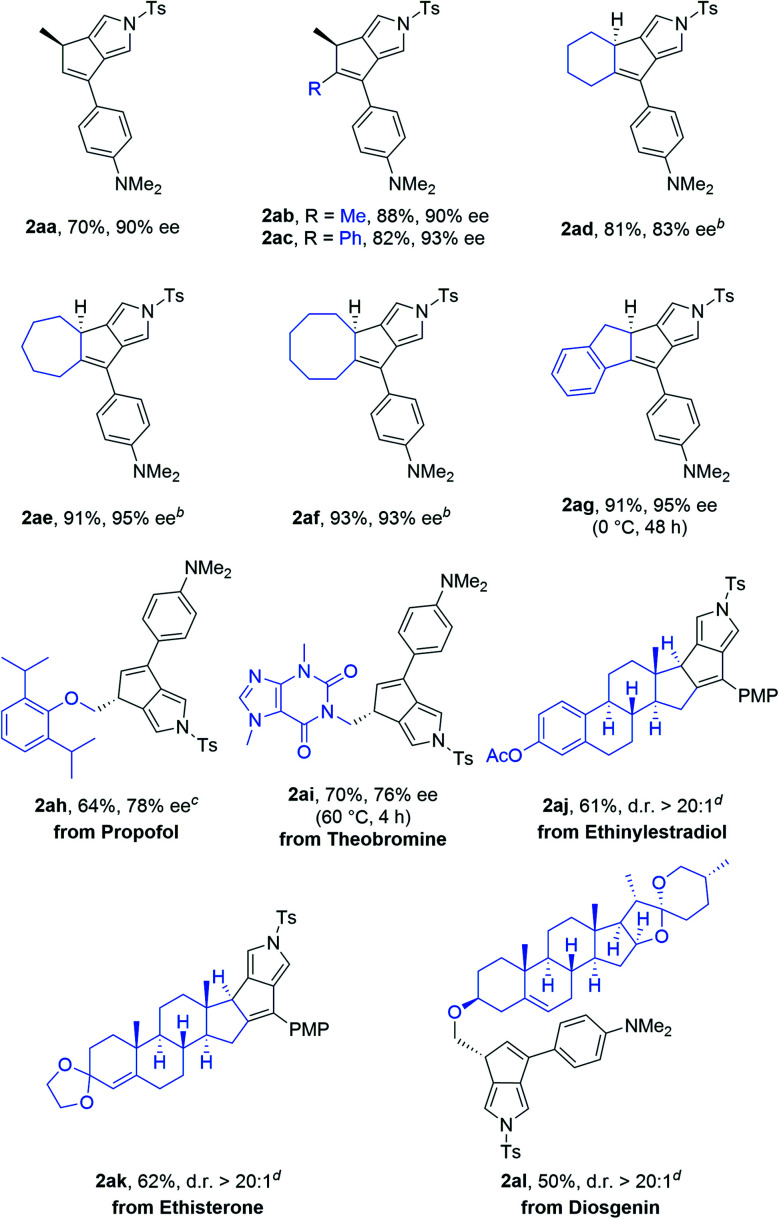
Scope of the asymmetric cyclization of alkenyl N-propargyl ynamides 1a.
|
|---|
|
Reaction conditions: 1 (0.2 mmol), Cu(MeCN)4PF6 (0.02 mmol), NaBArF4 (0.024 mmol), (S)-SEGPHOS (0.024 mmol), toluene (2 mL), 20 °C, 24 h, in Schlenk tubes; yields are those for the isolated products; ees are determined by HPLC analysis; d.r.s are determined by 1H NMR.
2-Me-THF as the solvent.
DCE as the solvent, 0 °C.
DCE as the solvent, 40 °C.
