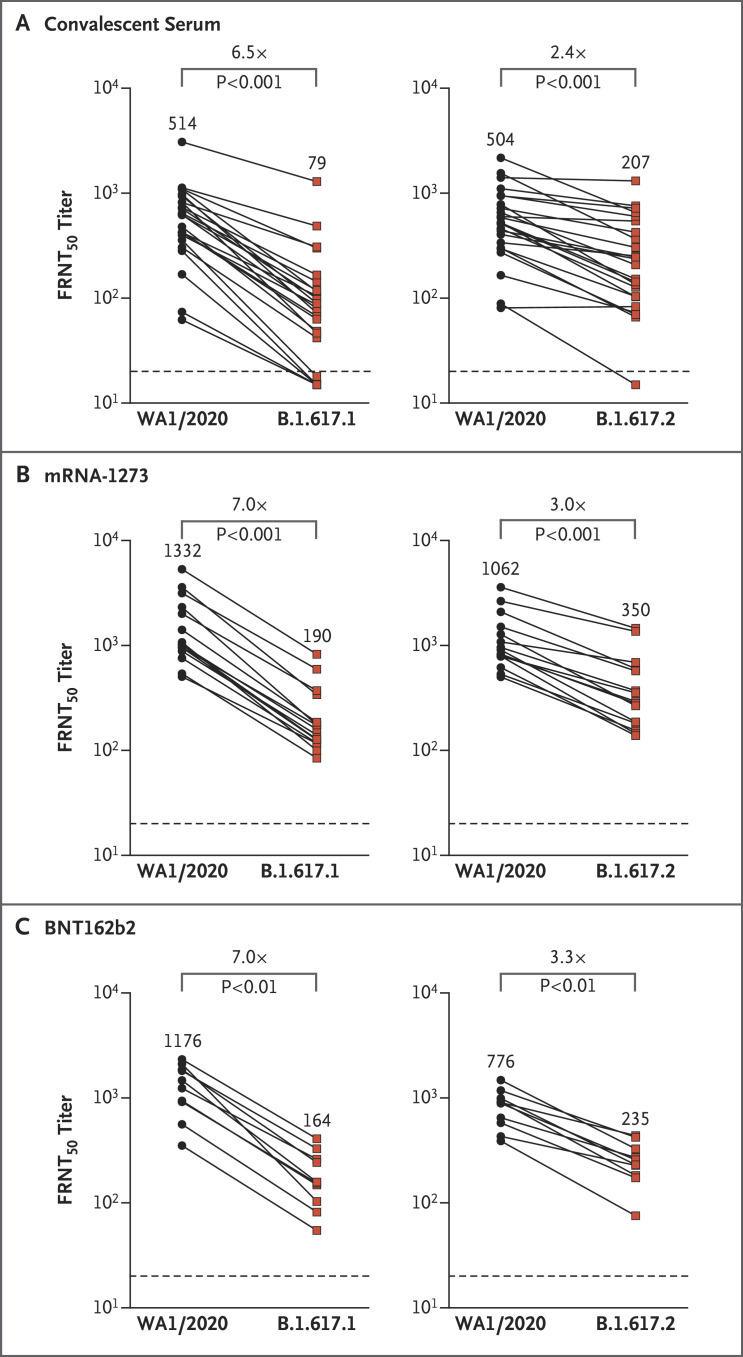
Figure 1. Neutralizing-Antibody Responses against the WA1/2020, B.1.617.1, and B.1.617.2 Variants.
Shown is the neutralizing activity against natural infection with severe acute respiratory syndrome coronavirus 2 among 24 samples from persons who had recovered from coronavirus disease 2019 (obtained 31 to 91 days after symptom onset) (Panel A), 15 samples from persons who had received the mRNA-1273 (Moderna) vaccine (obtained 35 to 51 days after the second dose) (Panel B), and 10 samples from persons who had received the BNT162b2 (Pfizer–BioNTech) vaccine (obtained 7 to 27 days after the second dose) (Panel C). Two independent neutralization assays were performed: activity against B.1.617.1 was compared with that against WA1/2020, and activity against B.1.617.2 was compared with that against WA1/2020. The focus reduction neutralization test (FRNT50 [the reciprocal dilution of serum that neutralizes 50% of the input virus]) geometric mean titers for WA1/2020, B.1.617.1, and B.1.617.2 are shown in each panel. The connecting lines between WA1/2020 and B.1.617.1 or WA1/2020 and B.1.617.2 represent matched serum samples. The horizontal dashed lines along the x axes indicate the limit of detection (FRNT50 geometric mean titer, 20). Normality of the data was determined with the use of the Shapiro–Wilk normality test. Nonparametric pairwise analyses for neutralization titers were performed with the use of the Wilcoxon matched-pairs signed-rank test.