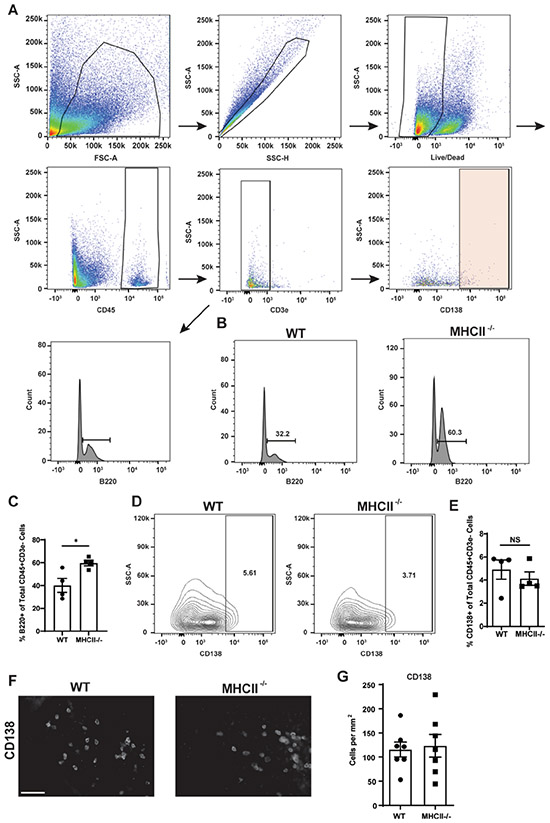
Fig. 4. PCs are present within the infarcts of MHCII−/− mice.
A.) Flow cytometry was performed on the infarcts of mice 7 weeks post-stroke. PCs were gated first by forward (FSC) and side (SSC) scatter to eliminate debris and then SSC area and SSC height to exclude doublets. Live/dead staining was then used to exclude dead cells. CD45 + staining was used to identify lymphocytes. CD3ε + cells (T-lymphocytes) were then excluded. B220 + or CD138 + was then used to determine the percentage of B-lymphocytes or PCs, respectively, in the remaining population. B.) Representative flow cytometry plots from the two mouse strains reveal an increased percentage of B-lymphocytes present within the CD45 + CD3ε-population in the infarct at 7 weeks post-DH stroke in MHCII−/− mice. C.) Quantification of the flow cytometry analysis of the percent B220 + cells out of the total CD45 + CD3ε- population. D.) Representative flow cytometry plots from the two mouse strains reveal a similar percentage of PCs present within the CD45 + CD3ε- population in the infarct of both WT and MHCII−/− mice at 7 weeks post-DH stroke. E.) Quantification of the flow cytometry analysis of the percent CD138 + cells out of the total CD45 + CD3ε- population. F.) Representative images of anti-CD138 immunostaining of brains sections from WT and MHCII−/− mice at 7 weeks post-DH stroke. Images are taken at 40x. Scale bar 100 μm. G.) Quantification of the number of CD138 + cells per mm2 in each of the strains revealed no difference between them in the number of PCs present within the infarct. n = 4 mice per group (flow cytometry), n = 7 mice per group (immunofluorescence). Data represent mean ± SEM. *p < 0.05 by Student’s t test.