Abstract
Objective:
This article reports the 3-year outcomes for the STePS multi-site Randomized Control Trial (RCT); reporting the overall impact of the STePS trial, and the differential impact of each arm of the trial (a resilience promoting intervention [PRP T1D] versus a diabetes education intervention [EI]) on diabetes-specific emotional distress and depressive symptoms.
Method:
Participants included 264 adolescents with type 1 diabetes (T1D), ages 14–18, in Chicago and San Francisco. Both intervention arms lasted 4.5 months and assessments were conducted at baseline, post-intervention (4.5 months), and five follow-up visits (8, 12, 16, 28 and 40 months from baseline). Intervention efficacy was investigated using latent growth curve modeling (LGCM) to analyze the rate and shape of change of outcomes from pre-intervention across post-intervention and follow-up time points.
Results:
Mean age of participants was 15.7 years, mean T1D duration was 6.9 years, mean HbA1c at baseline was 9.1%. The sample was diverse with nearly 35% identifying as racial or ethnic minorities, and 60% were female. PRP T1D participants reported significantly lower diabetes distress compared to EI participants, and the effect size increased over time. For the pooled sample, while 40% of youth reported elevated distress at baseline, only 23% reported elevated distress 3 years post-intervention. Moreover, PRP T1D participants experienced a significant decline in depressive symptoms from 16 to 40 months post-baseline, while participants in the education arm did not.
Conclusions:
Results from the three-year outcomes assessment demonstrate the robust effects of PRP T1D in adolescents with declines in distress and depressive symptoms.
Keywords: Distress, Depression, Adolescents, Type 1 Diabetes, Prevention
Depression is the most extensively investigated area of psychosocial functioning among youth with type 1 diabetes (T1D; Baucom et al., 2015; Hood et al., 2011; Matlock et al., 2017; McGrady & Hood, 2010). Teens with T1D are at increased risk for experiencing depressive symptoms, with prevalence rates ranging from 15% to 42%. In contrast, teens who do not have T1D show a prevalence rate between 0.4% and 8.3% (Rao & Chen, 2009). Moreover, teens with T1D and depressive symptoms show worse self-care behaviors and glycemic control (Baucom et al., 2015; Hilliard et al., 2016), more frequent emergency department visits and hospitalizations (Johnson et al., 2013), and worse quality of life (Hilliard et al., 2011). Diabetes-specific emotional distress is a similarly important construct for adolescents. Diabetes distress captures the worries, concerns and fears a person with diabetes experiences. It is an emotional response to the demands of living with diabetes. Both depression and distress are associated with worse metabolic outcomes in adolescents (Corathers et al., 2013; Hagger et al., 2018).
Few interventions have directly targeted adolescents with T1D experiencing poor behavioral or emotional functioning. One found that support group participation reduced depressive symptoms (Ellis et al., 2019). Another found a family-based intervention for youth with poor glycemic control improved depressive symptoms (Riley et al., 2015), and a third that focused on provider-patient discussions of quality of life showed no changes in depressive symptoms (de Wit et al., 2008). However, no studies exist that focus on preventing depressive symptoms or diabetes distress among youth with T1D. Our team adapted the Penn Resilience Program (PRP; Reivich et al., 2005), a well-established depression-prevention intervention, for use with teens with T1D. The Supporting Teen Problem Solving Study (STePS) is a randomized control study (RCT) comparing the adapted PRP program, which includes diabetes-specific content (PRP T1D; Hood et al., 2018; Weissberg-Benchell et al., 2016), to an advanced diabetes-education curriculum (EI).
The theoretical model for PRP blends both cognitive-behavioral and social problem-solving approaches for preventing depression. Cognitive risk factors implicated in depression are targeted, including: linking beliefs, feelings, and behaviors; identifying one’s own cognitive framework/thinking style; recognizing the impact of one’s thinking style on decision-making; and challenging negative thinking by evaluating the accuracy of one’s beliefs. Behavioral risk factors implicated in depression are also targeted, including problem-solving techniques such as negotiating, assertiveness, and decision-making; and coping skills such as relaxation techniques and seeking social support. The goal of the RCT was to test the efficacy of PRP T1D by comparing its effects to EI.
We hypothesized that PRP T1D would prevent depressive symptoms and diabetes distress among the participants. The original PRP studies showed increases in effect size on depressive symptom prevention over time. Specifically, the effect size post intervention was 0.09, and was 0.32 at 6 months (Gillham et al., 1994). Therefore, we hypothesized that the effect size for depressive symptoms and diabetes distress in PRP T1D would similarly increase over time. Further, we hypothesized that over time, the adaptive psychological functioning among the participants in the PRP T1D group would prevent sub-optimal glycemic control. This hypothesis is based on the fact that teens with high levels of distress (Hagger et al., 2018) and/or depressive symptoms (Corathers et al., 2013) experience worse glycemic control than their peers who do not experience these symptoms. Preventing diabetes distress is important, as data show that high levels of diabetes distress in adolescents is associated with poor glycemic (Abualula et al., 2018; Hagger et al., 2018) and psychosocial (Iturralde, Rauch et al., 2019; Iturralde, Weissberg-Benchell et al., 2017) outcomes.
EI was selected as the comparison group instead of standard diabetes care for two reasons: (1) to be consistent with a comparative effectiveness model for testing interventions and (2) to match participants for attention and time in intervention. We have published findings from the first year post-intervention assessment (Hood et al., 2018), which showed that all STePS participants experienced a significant reduction in diabetes-specific emotional distress, and those randomized to PRP T1D experienced significantly less distress one year post-treatment than those randomized to the EI group. Additional one-year findings showed that in both groups, depressive symptoms, resilience characteristics, and HbA1C values remained stable, and diabetes management behaviors deteriorated.
The aim of this article is to report 3-year outcomes for the STePS multi-site RCT. We report on the impact of participating in the STePS trial, and the differential impact of each arm of the trial (PRP T1D versus EI) on diabetes-specific emotional distress, depressive symptoms, diabetes management behaviors, glycemic control, and time in range. We hypothesized that youths receiving the PRP T1D intervention would continue to show lower levels of diabetes distress than those receiving EI over the three years since completing the intervention. We further hypothesized that those in the PRP T1D group would show lower levels of depressive symptoms over time compared to youths receiving EI. Finally, we hypothesized that those in the PRP T1D group would show improved self-care behaviors, glycemic control, and time in range compared to those receiving EI.
Method
STePS Program
The STePS study was an RCT with two arms: PRP T1D, which is the PRP curriculum adapted to include T1D specific challenges and examples; and EI, which covered prevailing topics in T1D, including nutrition for teenagers, the importance of exercise, a review of insulin action, and a review of diabetes technologies. Further details of the arms, including session focus and content, are provided in previous publications (Hood et al., 2018; Weissberg-Benchell et al., 2016). The PRP T1D intervention arm was led by masters-level clinicians. The EI arm was led by certified diabetes educators (CDE).
Interventionists for each arm worked from an Instructor’s Guide to help them proceed through the session topics and promote group discussion and interaction throughout. All participants were given an intervention-specific student workbook reviewing the concepts from each session. The workbook also contained homework reinforcing the concepts discussed each week. Both arms consisted of 9 sessions, scheduled twice per month, with groups of eight to twelve participants lasting 90–120 minutes. Active treatment lasted approximately 4.5 months.
Participants
Inclusion criteria for this study were 14–18 years of age, presence of T1D diagnosed according to American Diabetes Association criteria for at least one year, and daily insulin dosing of at least 0.5u/kg/day. Exclusion criteria included a current diagnosis of major depressive disorder or current treatment with an antidepressant. Further, adolescents with a psychotic disorder, major developmental disorder such as autism, or diagnosed eating disorder (e.g., anorexia nervosa) were excluded. In total, 264 adolescents with T1D were randomized, with 133 randomized to PRP T1D and 131 to EI. There were no differences in baseline demographic or clinical characteristics between the groups (ps > 0.05). The CONSORT diagram for the RCT is presented in Figure 1. Retention rates are reported in the Results section.
Figure 1.
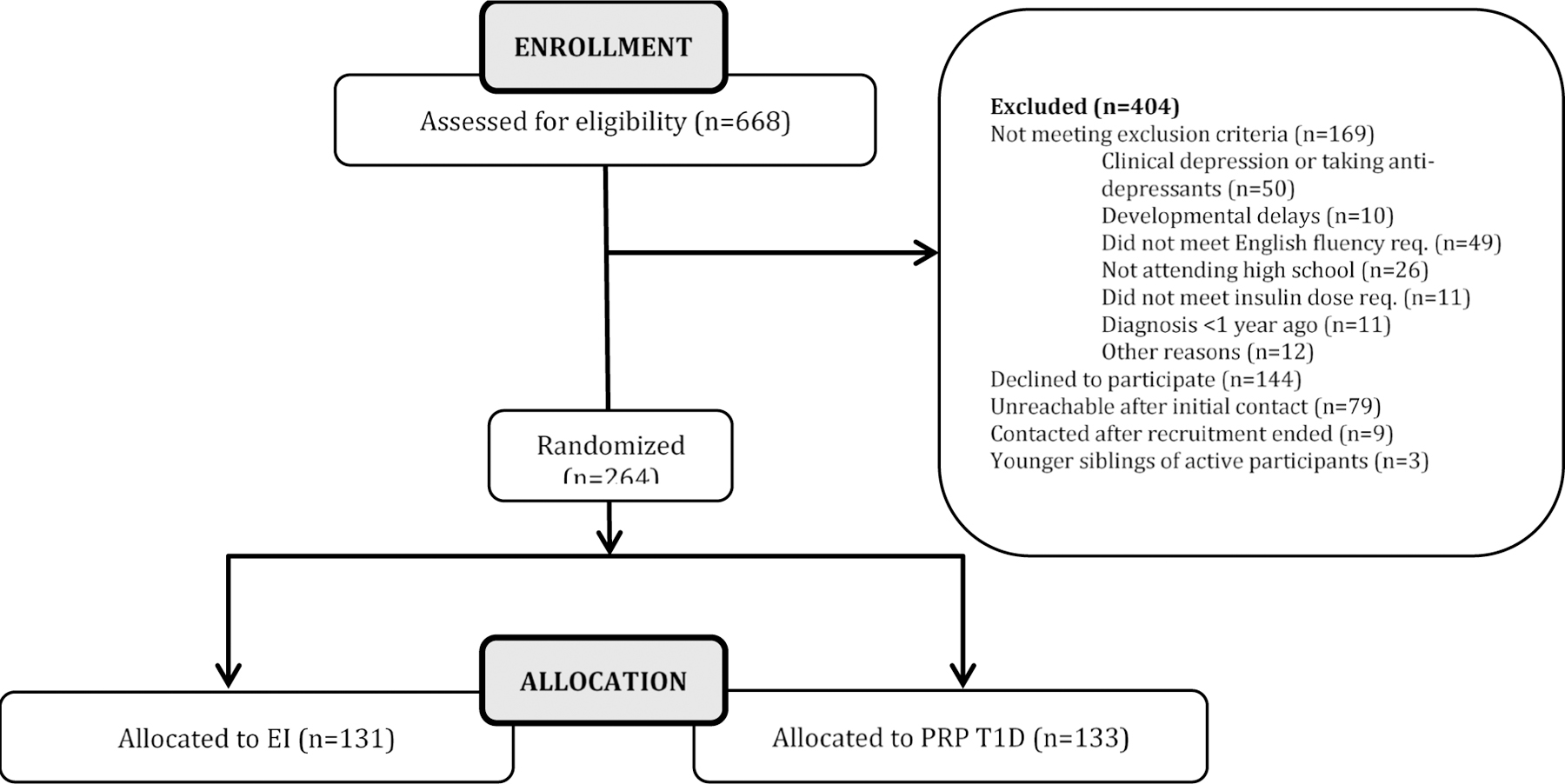
CONSORT Diagram
Study Visits & Procedures
The study had IRB approval at both study sites, with parents providing written consent and teens providing written assent at enrollment (and later consenting after they turned 18). The STePS program followed participants up to 3 years post-intervention. This report reviews exposure to the program and outcomes through all 3 years post-intervention. Data collection occurred at baseline (0 months), immediately at the end of the intervention (4.5 months), and then at 5 follow-up visits (8, 12, 16, 28 and 40 months from baseline; that is, up to 3 years post-treatment). At each study visit, questionnaires were completed electronically using HIPAA-compliant survey software, blood samples were collected for the HbA1c, and meter data were collected to calculate blood glucose monitoring frequency and time in range.
Measures
Diabetes Distress:
The Problem Areas in Diabetes – Teen (PAID-T) survey (Shapiro et al., 2018) assessed diabetes distress. Respondents rate how much each item currently applies to them over the past month using a 6-point scale (1 = not a problem to 6 = a serious problem). Example items include “feeling overwhelmed by the diabetes regimen,” and “feeling like my parents don’t trust me to care for my diabetes.” The PAID-T has 14 items, and baseline inter-item reliability was high across all time points (α = 0.93–0.94). Higher scores indicate more distress. The clinically significant cut-off score is ≥ 44 (Shapiro et al., 2018).
Depressive Symptoms:
The Children’s Depression Inventory (CDI; Kovacs, 2003) is a 27-item, widely used, psychometrically-strong measure of depressive symptoms. Youth rate each item on a scale from 0 to 2, based on perceived severity over the past 2 weeks. Higher scores indicate more depressive symptoms. Clinically significant cut-off scores are ≥ 15 for mild depressive symptoms and ≥ 20 for moderate depressive symptoms. The reliability for this sample was adequate (α = 0.86–0.92).
Diabetes Management:
The Self-Care Inventory - Revised (SCI-R; Lewin et al., 2009) offers a broad measure of diabetes management behaviors and tasks completed over the past 1–2 months. The SCI includes 14 items (α = 0.77–0.86). Blood glucose monitoring frequency was also obtained based on downloads of participant glucometer data over the past 14 days. Average daily checks were calculated by dividing the total number of checks from meter download by 14.
Glycemic Control:
A small fingerstick capillary sample of blood was collected at each assessment and sent to the central laboratory for processing. The central laboratory – Diabetes Diagnostic Laboratory at the University of Missouri – then provided a report of HbA1c. This method has been the gold standard metric of glycemic control since the 1990s.
Time in Range:
Percent time in range was calculated using blood glucose readings over the past 14 days downloaded from blood glucose meters. To maximize the amount of available data for calculating the percent of glucose readings within the recommended target range (70–180 mg/dL), data were included at each time point when participants had at least 14 checks over the 14 days (averaging at least one check per day). This cutoff was inclusive of participants whose average daily number of checks was 1 SD below the mean at baseline (M = 3.71, SD = 2.36).
Clinical and Sociodemographic Characteristics:
Adolescents and their primary caregivers provided information about gender, race/ethnicity, family income, and educational attainment of caregivers. Chart review was conducted to obtain diabetes duration, insulin delivery regimen, and pre-screening eligibility criteria.
Analytic Plan
Intervention efficacy across the seven time-points was investigated using latent growth curve modeling (LGCM) via Mplus version 8.1 software (Muthen & Muthen, 2017) to analyze the rate and shape of change of outcomes from pre-intervention across post-intervention and follow-up time points. Models were first run combined across intervention and control groups, and then intervention group assignment was added as a dummy-coded (EI = 0, PRP T1D = 1) exogenous time-invariant covariate to assess the mean difference between groups (Bollen & Curran, 2006). Four criteria were used to determine acceptable model fit: (1) root mean square error of approximation (RMSEA < .08; Browne & Cudeck, 1992); (2) comparative fit index (CFI > .90; Marsh et al., 2004); (3) Tucker-Lewis index (TLI > .90; Marsh et al., 2004); and (4) standardized root mean square residual (SRMR < .08; Hu & Bentler, 1998). For each outcome (diabetes-specific distress, depressive symptoms, glycemic control, blood glucose monitoring frequency, diabetes management behaviors, and time in range), separate LGCMs were estimated.1 Missing data were accounted for using full-information robust maximum likelihood estimation, which uses all available data to estimate the model parameters by deriving model fit from a summation of fit functions for each case (Enders, 2010).
Because preliminary examination of the data indicated possible non-linear patterns, intercept-only, linear, quadratic (possible curvilinear change), cubic (possible S-curve shaped changes), and latent basis with freed factor-loadings were tested. In addition, linear-linear piecewise models (two segments of change depicting different linear slopes), and quadratic-linear piecewise models (two segments of change in which one segment shows curvilinear change and another segment shows linear change) were tested. The two segments of piecewise models were joined at a knot point, or point at which one change segment transitions to another segment of change, which was set at a specific time point based on visual inspection of change in mean values of the tested outcome. The various estimated models were used to identify change in outcomes as accurately as possible given that there may be greater nuance in change than simple linear patterns alone may capture.
To identify the best-fitting model, nested models with adequate fit were contrasted with relevant “comparison” models using the Satorra-Bentler scaled chi-square difference test (ΔSBχ2, Bryant & Satorra, 2012), with a significant chi-square difference value indicating that the more parsimonious, nested model with fewer parameters fits the data significantly worse, compared to the less parsimonious, comparison model. A nested model is a model that contains a subset of the parameters of another more complex “comparison” model, and is a requirement when using chi-square difference testing for model comparison. To identify the best-fitting model among non-nested models, the Akaike Information Criterion (AIC; Akaike, 1987), Bayesian information criteria (BIC; Schwarz, 1978), and sample-size adjusted BIC (SABIC; Sclove, 1987) were used, with lower values indicating better fit.
For the linear model, the intercept was set at the final time-point (40 months post-baseline, which translated to roughly three years post-intervention) by specifying the zero point of the slope factor at the last time-point. The loadings for the linear slope factor across the seven respective time-points were fixed to −10, −8.875, −8, −7, −6, −3, and 0, consistent with the time in months between assessments, with a change of one unit in time equal to four months (see Figure 2). Once the best-fitting model was identified, group assignment (PRP T1D versus EI) was added to the model as an exogenous predictor of both the intercept (set to three years post-intervention) and slope (e.g, linear, quadratic) factors. The main effect of dose (number of sessions attended) and interaction effect of dose x intervention group were also investigated as predictors of intercept and slope factors. Significant effects were examined using the MODEL CONSTRAINT and LOOP PLOTS commands to analyze simple slopes (Curran et al., 2004).
Figure 2. Linear Latent Growth Curve Model of Change in the Outcome Variable Across Seven Time-Points.
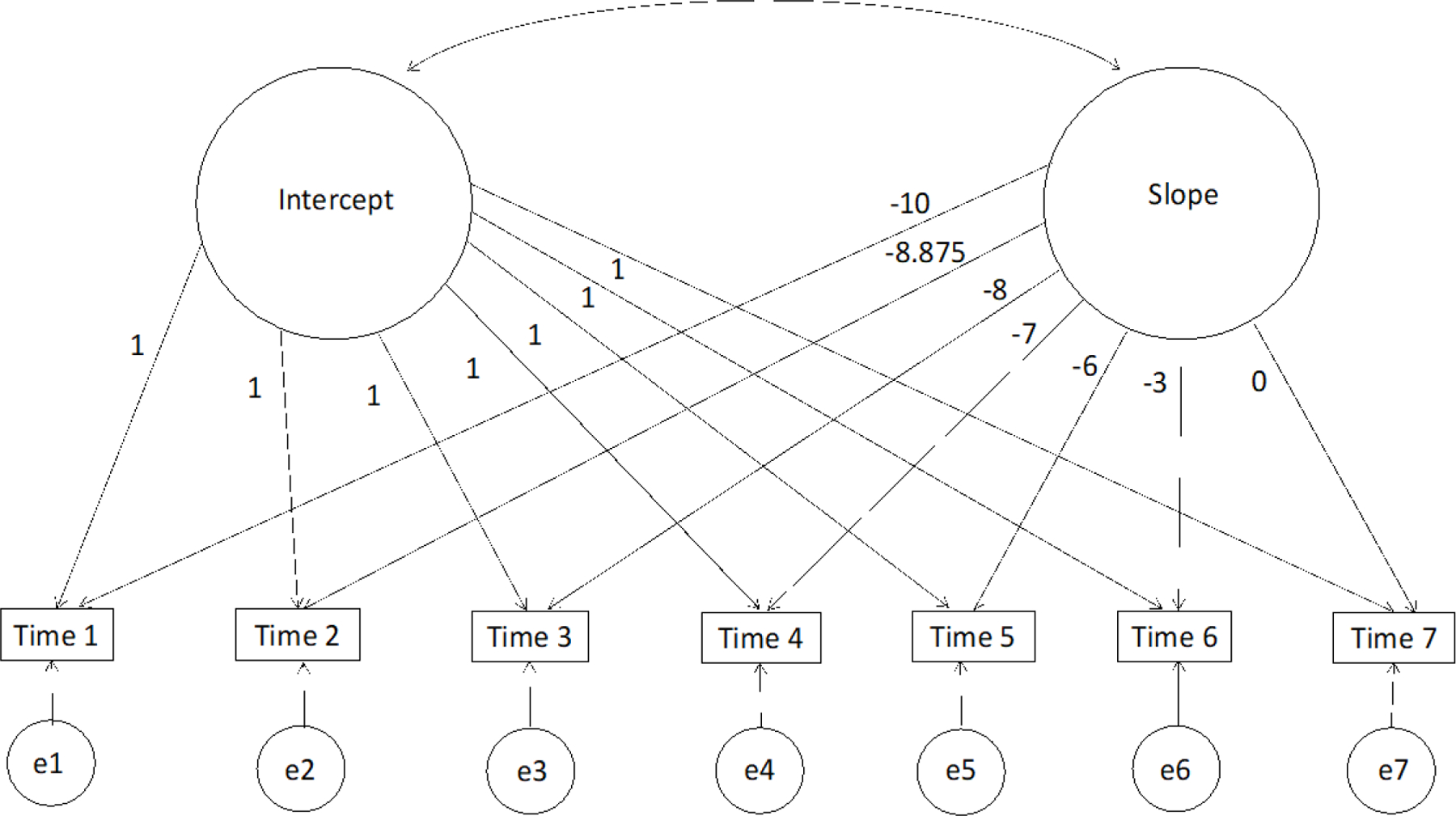
Note. LGCMs or curve-of-factor models were evaluated separately for each outcome variable (i.e., diabetes-specific distress, depressive symptoms, glycemic control, blood glucose monitoring frequency, diabetes management behaviors, time in range). The intercept was set at the final time-point (40 months post-baseline) by specifying the zero point of the slope factor at T7. The loadings of the linear slope factor across the seven respective time-points were set to the amount of time between assessment time-points (−10, −8.875, −8, −7, −6, −3, 0 months), with a one-unit change in time equal to four months.
Results
Table 1 displays participant characteristics, and Table 2 presents means and standard deviations for all study variables. The sample had a mean age of M = 15.7 years (SD = 1.1), mean T1D duration of M = 6.9 years (SD = 4.0), and HbA1c at baseline of M = 9.1% (SD = 1.9). The sample included more females (60%) than males and was more diverse than national norms of teens with T1D in that nearly 35% identified as racial or ethnic minorities (Miller et al., 2015).
Table 1.
Participant Characteristics (N = 264)
| Baseline Variables | N | % | M | SD |
|---|---|---|---|---|
| Age (years) | 15.74 | 1.09 | ||
| Diabetes duration (years) | 6.88 | 4.03 | ||
| Hemoglobin A1C (%) | 9.14 | 1.92 | ||
| Blood glucose monitoring frequency (Daily average over past 14 days) | 3.71 | 2.36 | ||
| Gender | ||||
| Male | 106 | 40.2 | ||
| Female | 158 | 59.8 | ||
| Race or ethnicity | ||||
| White, non-Hispanic | 173 | 65.5 | ||
| African American | 38 | 14.4 | ||
| Hispanic | 29 | 11.0 | ||
| Asian or Pacific Islander | 6 | 2.3 | ||
| Native American or Alaska Native | 3 | 1.1 | ||
| Reported as “Other” | 15 | 5.7 | ||
| Family income | ||||
| Less than $50,000 | 39 | 14.8 | ||
| $50,000-$75,000 | 38 | 14.4 | ||
| $76,000-$100,000 | 43 | 16.3 | ||
| $101,000-$150,000 | 50 | 18.9 | ||
| More than $150,000 | 63 | 23.9 | ||
| Not reported | 19 | 7.2 | ||
| Mother’s education (college graduate) | 162 | 61.4 | ||
| Two-parent home | 158 | 59.8 | ||
| Intervention assignment | ||||
| Resilience | 133 | 50.4 | ||
| Education | 131 | 49.6 | ||
| Insulin regimen at baseline | ||||
| Injections | 79 | 29.9 | ||
| Insulin pump | 185 | 70.1 | ||
| CGM use at baseline | 80 | 31.3 |
Table 2.
Means and Standard Deviations of Study Variables
| Measure | Baseline M (SD) |
4 mos M (SD) |
8 mos M (SD) |
12 mos M (SD) |
16 mos M (SD) |
28 mos M (SD) |
40 mos M (SD) |
|---|---|---|---|---|---|---|---|
| PAID-T | 40.35 (15.90) N=260 |
38.03 (15.43) N=229 |
37.44 (16.19) N=237 |
35.75 (16.23) N=240 |
35.92 (15.89) N=225 |
35.69 (16.49) N=231 |
34.28 (16.29) N=229 |
| CDI | 7.72 (6.17) N=260 |
7.82 (6.95) N=229 |
7.82 (7.18) N=237 |
7.87 (7.90) N=240 |
8.49 (8.23) N=225 |
7.58 (7.57) N=231 |
7.42 (7.53) N=229 |
| HbA1c | 9.14 (1.92) N=264 |
9.07 (1.97) N=232 |
9.03 (1.86) N=225 |
9.07 (1.87) N=230 |
9.19 (1.89) N=226 |
9.27 (2.05) N=226 |
9.21 (1.91) N=207 |
| Blood Glucose Monitoring Frequency | 3.71 (2.36) N=236 |
3.51 (2.24) N=221 |
3.54 (2.11) N=219 |
3.49 (2.22) N=228 |
3.13 (2.25) N=204 |
2.98 (2.22) N=211 |
2.79 (2.09) N=195 |
| SCI-R | 65.10 (13.08) N=260 |
65.18 (13.80) N=229 |
63.61 (15.45) N=237 |
62.58 (15.39) N=240 |
62.69 (15.77) N=225 |
61.90 (16.06) N=231 |
60.28 (17.24) N=229 |
| Time in Range | 37.84 (17.34) N=213 |
38.58 (15.89) N=195 |
38.14 (16.55) N=200 |
38.21 (17.49) N=203 |
37.19 (17.54) N=165 |
35.94 (17.21) N=167 |
35.58 (15.95) N=126 |
Note. PAID-T, Problem Areas in Diabetes-Teen Version; CDI, Children’s Depression Inventory; HbA1c, Hemoglobin A1c; SCI-R, Self-Care Inventory-Revised.
The sample size for each key study outcome at various waves of data collection is displayed in Table 2. While retention rates remained very high throughout the study (88–92% across follow-up time points), there were minimal missing data due to participants either not completing every assessment or only completing self-report while not providing objective data from meter downloads and blood draws. Little’s Test was used to assess the assumption of data missing completely at random (MCAR) for study outcomes. Data were not MCAR for HbA1c, χ2 (105, N = 264) = 152.84, p = .002, self-reported diabetes management, χ2 (133, N = 264) = 167.41, p = .023, depressive symptoms, χ2 (133, N = 264)=165.94, p = .028, or diabetes distress, χ2(133, N = 264) = 197.92, p = .000. The proportion of missing responses did not differ based on intervention group at any time point, except for blood sugar checks at 16-months post-baseline, χ2(1, N = 264) = 5.21, p = .022 (less data for PRP T1D [24% missing data] compared to EI [16% missing data]). Missing data did not differ based on baseline values, except for HbA1c, such that higher HbA1c at baseline was associated with more missing data, F(1, 262) = 9.28, p = .003.
Exposure to Treatment and Retention Rates
The average number of sessions attended approached 7 (M = 6.85, SD = 3.05) and there were no differences between groups on number of sessions attended. Nearly two-thirds of participants completed all of the treatment sessions (1–8; session 9 was review). Retention at one-year post-treatment was 92%, at two-years was 92%, and at three-years was 88%. Only 4 participants formally dropped out of the study before the intervention was completed, and an additional 7 dropped out of ongoing data collection. The rest were unable to be reached.
Intercept-only, linear, quadratic, cubic, latent basis, and piecewise latent growth curve models were evaluated for each outcome (see Table 3). None of the cubic models for any of the outcomes converged such that the pattern of nonconvergence suggested that the cubic models were untenable representations of temporal change in each of the outcome measures. Table 4 shows results of intervention group as a predictor of both the outcome variable at 40 months post-baseline (intercept) and the rate of change (e.g., linear/quadratic slope).
Table 3.
Goodness-of-Fit Statistics for Longitudinal Models of Change in Study Outcomes Collapsed Across PRP T1D and Diabetes Education Groups
| Type of model | RMSEA | CFI | TLI | SRMR | AIC | BIC | SABIC | SBχ2 | df |
|---|---|---|---|---|---|---|---|---|---|
| Diabetes Distress (N=264) | |||||||||
| Intercept-only | 0.13 | 0.84 | 0.87 | 0.10 | 12806.23 | 12838.45 | 12809.92 | 143.49 | 26 |
| Linear | 0.08 | 0.94 | 0.95 | 0.08 | 12706.88 | 12749.83 | 12711.79 | 64.82 | 23 |
| Quadratica | 0.04 | 0.99 | 0.99 | 0.05 | 12667.10 | 12720.79 | 12673.24 | 29.71 | 20 |
| Latent Basis | 0.08 | 0.94 | 0.95 | 0.08 | 12706.15 | 12749.11 | 12711.06 | 64.25 | 23 |
| Linear-Linear Piecewise (Knot = 12 months) | 0.04 | 0.99 | 0.99 | 0.04 | 12665.98 | 12723.26 | 12672.53 | 27.77 | 19 |
|
Depressive Symptoms (N=264) | |||||||||
| Intercept-only | 0.12 | 0.86 | 0.89 | 0.10 | 10207.96 | 10240.18 | 10211.64 | 117.88 | 26 |
| Linear | 0.10 | 0.91 | 0.91 | 0.09 | 10167.07 | 10210.03 | 10171.98 | 85.05 | 23 |
| Quadratic | 0.07 | 0.97 | 0.96 | 0.06 | 10116.98 | 10174.26 | 10123.53 | 41.89 | 19 |
| Latent Basis | 0.12 | 0.90 | 0.89 | 0.06 | 10146.96 | 10207.81 | 10153.92 | 81.96 | 18 |
| Linear-Linear Piecewise (Knot = 12 months) | 0.00 | 1.00 | 1.01 | 0.08 | 10816.57 | 10873.85 | 10823.12 | 14.86 | 19 |
| Quadratic-Linear Piecewise (Knot = 16 months) | 0.03 | 1.00 | 0.99 | 0.03 | 10090.29 | 10165.47 | 10098.89 | 16.72 | 14 |
|
Glycemic Control (N=264) | |||||||||
| Intercept-only | 0.13 | 0.86 | 0.89 | 0.11 | 5099.31 | 5131.53 | 5102.99 | 136.73 | 26 |
| Linear | 0.10 | 0.93 | 0.93 | 0.11 | 5022.14 | 5065.10 | 5027.05 | 79.76 | 23 |
| Quadratica | 0.08 | 0.95 | 0.95 | 0.09 | 4988.11 | 5041.81 | 4994.25 | 56.61 | 20 |
| Latent Basisb | 0.13 | 0.88 | 0.88 | 0.11 | 5090.02 | 5143.72 | 5096.16 | 110.27 | 20 |
| Linear-Linear Piecewise (Knot = 12 months) | 0.06 | 0.98 | 0.98 | 0.09 | 4956.50 | 5013.77 | 4963.04 | 36.39 | 19 |
| Quadratic-Linear Piecewise (Knot = 16 months) | 0.06 | 0.98 | 0.98 | 0.04 | 4947.78 | 5022.95 | 4956.37 | 26.39 | 14 |
|
Blood Glucose Monitoring Frequency (with CGM used as time-varying covariate, N=264) | |||||||||
| Intercept-Only | 0.09 | 0.82 | 0.80 | 0.06 | 7341.84 | 7549.46 | 7365.57 | 180.95 | 61 |
| Linear | 0.07 | 0.91 | 0.87 | 0.04 | 7271.83 | 7515.25 | 7299.65 | 114.66 | 51 |
| Quadratic | 0.07 | 0.92 | 0.86 | 0.03 | 7253.87 | 7536.67 | 7286.20 | 94.55 | 40 |
| Latent Basis | 0.05 | 0.96 | 0.94 | 0.04 | 7200.09 | 7461.41 | 7229.96 | 75.20 | 46 |
| Linear-Linear Piecewise (Knot = 12 months) | 0.07 | 0.92 | 0.85 | 0.04 | 7275.13 | 7557.93 | 7307.46 | 96.52 | 40 |
| Quadratic-Linear Piecewise (Knot = 16 months)b | 0.05 | 0.96 | 0.93 | 0.03 | 7213.63 | 7500.01 | 7246.36 | 65.37 | 39 |
|
Diabetes Management Behaviors (N=264) | |||||||||
| Intercept-Only | 0.10 | 0.89 | 0.91 | 0.21 | 12602.72 | 12634.94 | 12606.40 | 92.22 | 26 |
| Linear | 0.06 | 0.97 | 0.97 | 0.15 | 12532.60 | 12575.56 | 12537.51 | 41.16 | 23 |
| Quadratic | 0.02 | 1.00 | 1.00 | 0.09 | 12512.54 | 12569.82 | 12519.09 | 21.39 | 19 |
| Latent Basis | 0.05 | 0.98 | 0.98 | 0.09 | 12524.11 | 12584.96 | 12531.06 | 28.24 | 18 |
| Linear-Linear Piecewise (Knot = 12 months) | 0.00 | 1.00 | 1.01 | 0.06 | 12503.32 | 12560.60 | 12509.87 | 14.86 | 19 |
| Quadratic-Linear Piecewise (Knot = 16 months) | 0.00 | 1.00 | 1.02 | 0.03 | 12500.42 | 12575.60 | 12509.02 | 5.97 | 14 |
|
Time in Range (N=252) | |||||||||
| Intercept-Only | 0.08 | 0.90 | 0.92 | 0.08 | 10335.47 | 10367.23 | 10338.70 | 67.47 | 26 |
| Linear | 0.05 | 0.96 | 0.97 | 0.07 | 10312.03 | 10354.38 | 10316.34 | 39.06 | 23 |
| Quadratic | 0.05 | 0.97 | 0.97 | 0.06 | 10312.52 | 10368.99 | 10318.27 | 32.01 | 19 |
| Latent Basis | 0.08 | 0.94 | 0.93 | 0.06 | 10316.06 | 10376.06 | 10322.17 | 43.89 | 18 |
| Linear-Linear Piecewise (Knot = 12 months) | 0.04 | 0.98 | 0.98 | 0.06 | 10307.01 | 10363.48 | 10312.76 | 27.18 | 19 |
| Quadratic-Linear Piecewise (Knot = 16 months) | 0.05 | 0.98 | 0.97 | 0.06 | 10312.95 | 10387.07 | 10320.49 | 23.23 | 14 |
Note. N = 264 (PRP T1D: N = 133; EI: N = 131). Adequate fit indicated by RMSEA < .08, CFI > .90, TLI > .90, SRMR < .08.
T7 residual variance fixed to zero.
Variance of the first linear factor fixed to zero.
Table 4.
Intervention group as a predictor of outcomes at three years post-intervention (40 months post-baseline) and the rate of change in outcomes over time
| Outcome | b | SE | β | p |
|---|---|---|---|---|
| Diabetes Distress | ||||
| Intercept (3 Years Post-Intervention) | −5.28** | 2.03 | −0.17 | .009 |
| Linear Slope 1 (0–12 months) | −0.68 | 0.53 | −0.11 | .204 |
| Linear Slope 2 (12–40 months) | −0.37 | 0.26 | −0.11 | .146 |
|
Depressive Symptoms | ||||
| Intercept (3 Years Post-Intervention) | −1.76 | 0.94 | −0.13 | .060 |
| Linear Slope 1 (0–16 months) | −0.04 | 0.58 | −0.01 | .942 |
| Quadratic Factor (0–16 months) | −0.03 | 0.14 | −0.02 | .838 |
| Linear Slope 2 (16–40 months) | −0.30* | 0.15 | −0.18 | .040 |
|
Glycemic Control | ||||
| Intercept (3 Years Post-Intervention) | 0.21 | 0.27 | 0.05 | .428 |
| Linear Slope 1 (0–16 months) | 0.18 | 0.12 | 0.13 | .137 |
| Quadratic Factor (0–16 months) | 0.04 | 0.03 | 0.10 | .233 |
| Linear Slope 2 (16–40 months) | 0.01 | 0.03 | 0.02 | .767 |
|
Blood Glucose Monitoring Frequency | ||||
| Intercept (3 Years Post-Intervention) | −0.24 | 0.25 | −0.06 | .347 |
| Linear Slope | −0.02 | 0.02 | −0.07 | .395 |
|
Diabetes Management Behaviors | ||||
| Intercept (3 Years Post-Intervention) | 1.99 | 2.17 | 0.07 | .358 |
| Linear Slope 1 (0–12 months) | 0.63 | 0.51 | 0.12 | .211 |
| Linear Slope 2 (12–40 months) | 0.31 | 0.25 | 0.14 | .217 |
|
Time in Range | ||||
| Intercept (3 Years Post-Intervention) | −1.70 | 2.45 | −0.07 | .488 |
| Linear Slope 1 (0–12 months) | 0.30 | 0.73 | 0.05 | .679 |
| Linear Slope 2 (12–40 months) | −0.39 | 0.36 | −0.15 | .273 |
Note. PRP T1D: N = 133; EI: N = 131.
p < .05.
p < .01.
Diabetes Distress2
The quadratic and linear-linear piecewise models provided adequate fit to the diabetes distress data across fit-indices (see Table 3). Because these models were not nested, ΔSBχ2 could not be used to compare their goodness-of-fit. The piecewise model fit slightly better than the quadratic model based on AIC and SABIC, but not based on BIC. Given similarities in fit, the piecewise model with a knot at 12 months was selected as the most appropriate model, in order to allow for more nuanced interpretation of change in distress and predictors of change before and after the knot point (see Figure 3). The piecewise model showed a linear decline in diabetes distress from 0 to 12 months (slope1: b = −1.39, SE = 0.27, p < .001, ß = −0.46), followed by stabilization in diabetes distress from 12 to 40 months (slope2: b = −0.23, SE = 0.13, p = .083, ß = −0.14). The effect size of change in diabetes distress from 0 to 12 months was d = 0.26, calculated following Feingold’s (2009) recommendations. The model’s baseline rating of diabetes distress was 40.38 (SE = 0.96, p < .001, ß = 2.88), and the final mean rating of diabetes distress at 40 months was 34.60 (SE = 1.04, p < .001, ß = 2.27). Intervention group predicted diabetes distress at three years post-intervention (intercept factor), b = −5.28, SE = 2.03, p = .009, β = −0.17, d = 0.32, such that PRP T1D participants reported significantly lower diabetes distress (M = 31.70, SD = 15.16) compared to participants in EI (M = 36.94, SD = 16.83).
Figure 3. Piecewise Model Depicting Change in Diabetes Distress from Pre-Intervention to 40 Months Post-Baseline (About Three Years Post-Intervention) Separately for Each Intervention Group (PRP T1D: N = 133; EI: N = 131).
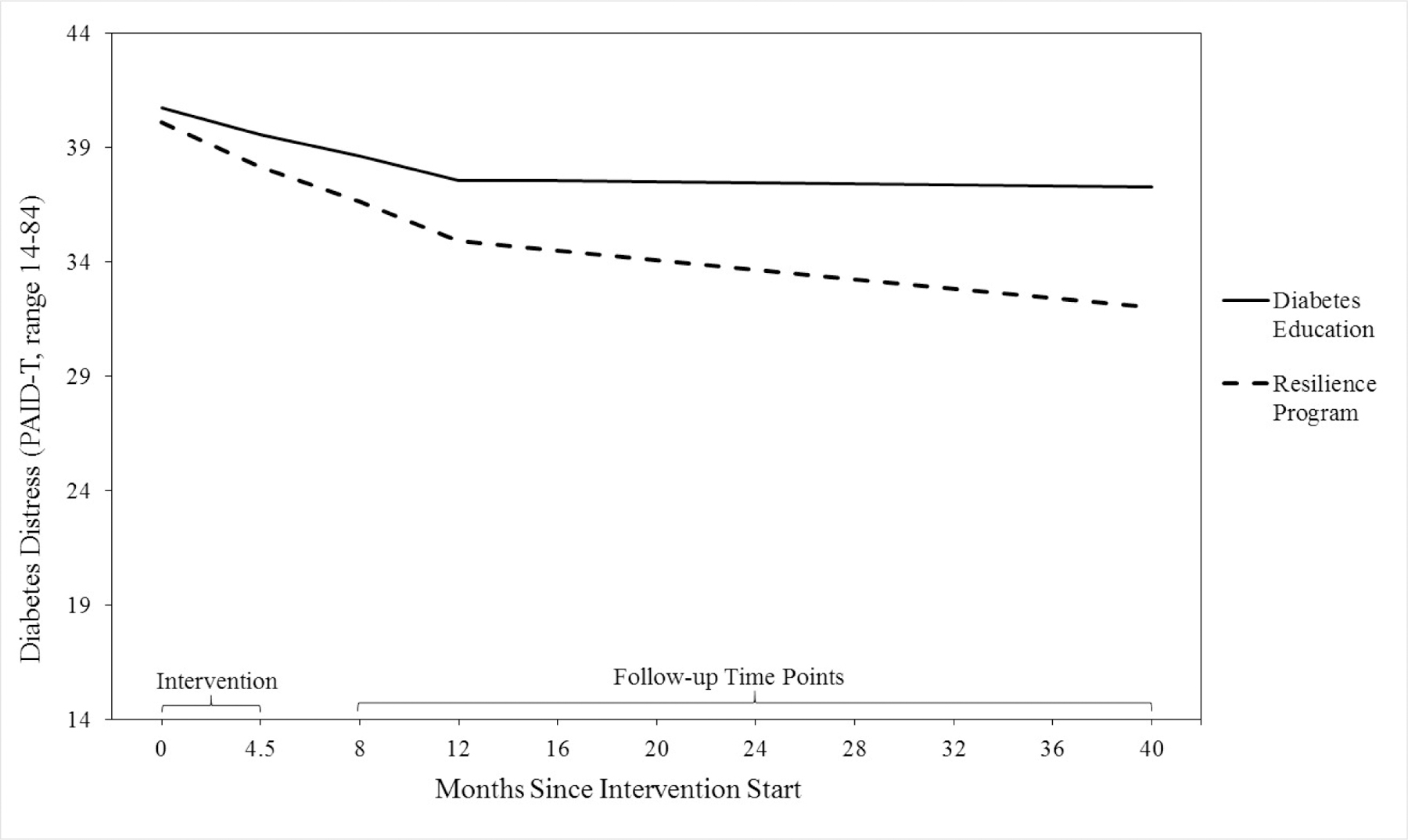
Note. The clinical cut-off score for diabetes distress is > 44 (Shapiro et al., 2018).
To further assess change in effect sizes based on intervention group, the intercept of the best-fitting LGCM was set at each of the seven assessment time points from baseline to forty months post-baseline, and intervention group was added as a predictor of each intercept value. The effect size increased over time, such that there was a non-significant post-intervention effect at 4 months post-baseline (β = −0.05, b = −1.40, SE = 1.73, p = .419), and increases in effects over time (8 months: β = −0.07, b = −1.99, SE = 1.73, p = .250; 12 months: β = −0.09, b = −2.67, SE = 1.88, p = .156; 16 months: β = −0.11, b = −3.04, SE = 1.80, p = .090; 28 months: β = −0.15, b = −4.16, SE = 1.76, p = .018; 40 months: β = −0.17, b = −5.28, SE = 2.03, p = .009).
Regarding clinical significance of improvements in diabetes distress, there was a difference in rates of elevated distress at 3 years post-intervention compared to baseline (pooled across both PRP T1D and EI), evaluated using the McNemar χ2 test, p < .001. Specifically, these findings indicate that 40% of youth experienced elevated distress at baseline, compared to only 23% at 3 years post-intervention. Further, the odds of a clinically significant reduction in distress at 3-year follow-up for teens who reported elevated distress at baseline were 6.5 times greater than the increase in distress for teens who did not report elevated distress at baseline. This odds ratio is consistent with a large effect size, Cohen’s d > 0.8 (Chen et al., 2010). Thus, 52% of participants with elevated distress at baseline no longer met criteria for elevated distress at 3 years post-intervention, whereas only 13% of participants without elevated distress at baseline endorsed elevated distress at 3 years post-intervention. These findings suggest that there were statistically and clinically significant improvements in diabetes distress over 3 years, combined across intervention groups.
Depressive Symptoms3
The quadratic model and quadratic-linear piecewise model with a knot at 16 months provided adequate fit to the depressive symptoms data (see Table 3). ΔSBχ2 could not be used to compare models’ goodness-of-fit because models were not nested. The quadratic-linear piecewise model fit the data best based on AIC, BIC, and SABIC, and was therefore selected as most appropriate. This model showed stable depressive symptoms from 0 to 16 months (slope1: b = 0.41, SE = 0.28, p = .139, ß = 0.16; quadratic slope b = 0.07, SE = 0.07, p = .269, ß = 0.10), followed by a decline in depressive symptoms from 16 to 40 months (slope2: b = −0.17, SE = 0.07, p = .018, ß = −0.20). The effect size of change in depressive symptoms from 16 to 40 months was d = 0.12 (Feingold, 2009). The model’s baseline mean depressive symptom rating was 7.74 (SE = 0.39, p < .001, ß = 1.29) and final rating was 7.22 (SE = 0.46, p < .001, ß = 1.05).
Intervention group significantly predicted change in depressive symptoms such that PRP T1D participants experienced a more rapid decline in depressive symptoms from 16 to 40 months post-baseline, compared to participants in EI, b = −0.30, SE = 0.15, p = .040, β = −0.18. The treatment-slope effect was d = 0.22. Follow-up assessment of change in depressive symptoms from 16 to 40 months separated by intervention group indicated that there was a significant decline in depressive symptoms for the PRP T1D participants, b = −0.31, SE = 0.11, p = .005, β = −0.31, but not for EI participants, b = −0.01, SE = 0.09, p = .936, β = −0.01 (see Figure 4). There was also a marginally significant difference in depressive symptoms at 40 months, b = −1.76, SE = 0.94, p = .060, β = −0.13, d = 0.23, such that PRP T1D participants (M = 6.73, SD = 6.77) reported fewer depressive symptoms compared to participants in EI (M = 8.13, SD = 8.11).
Figure 4. Piecewise Model Depicting Change in Depressive Symptoms from Pre-Intervention to 40 Months Post-Baseline (Roughly Three Years Post-Intervention) Separately for Each Intervention Group (PRP T1D: N = 133; EI: N = 131).
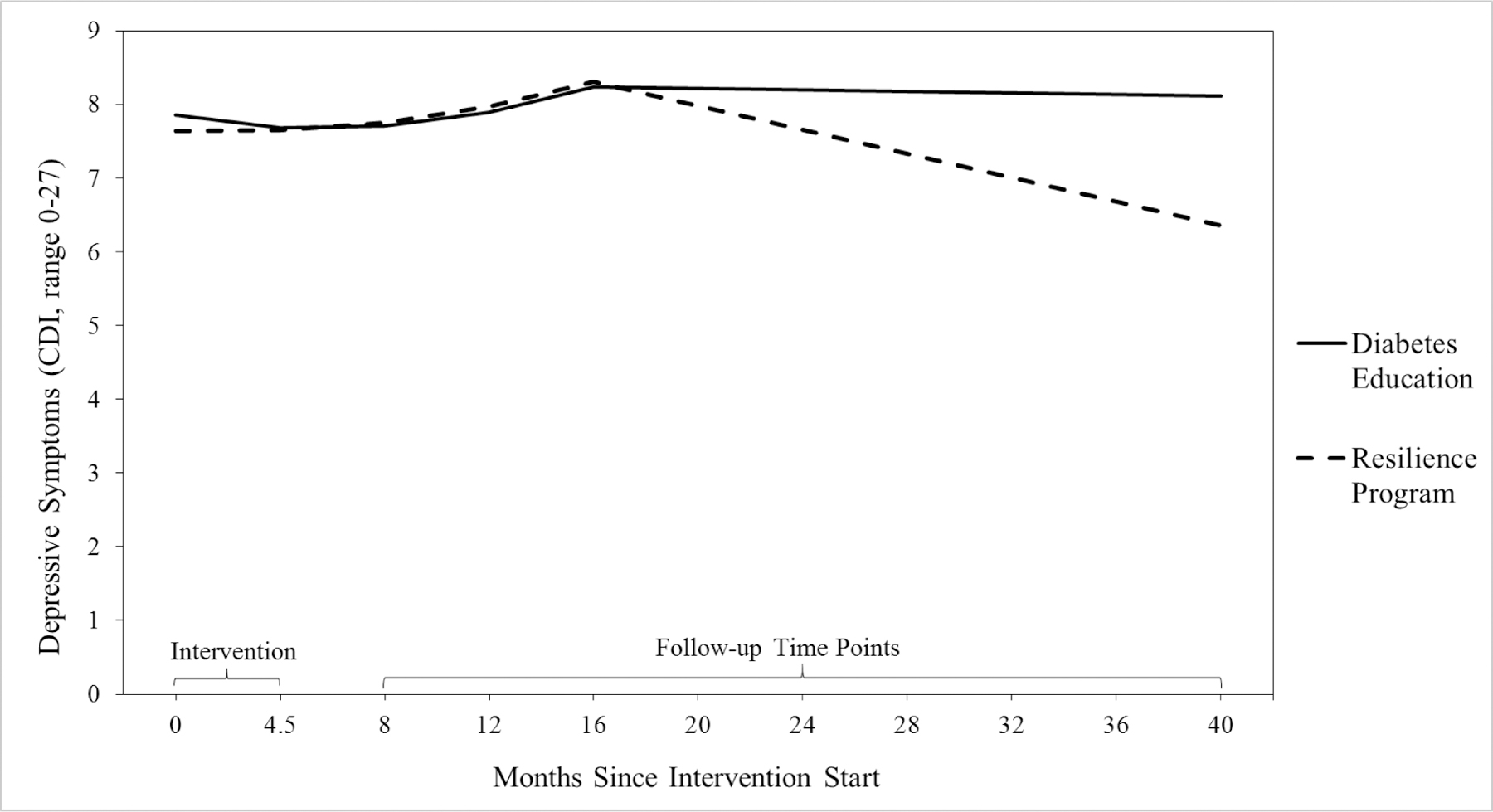
Note. Clinical cut-off scores for depressive symptoms are ≥ 15 for mild depressive symptoms and ≥ 20 for moderate depressive symptoms (Kovacs, 2003).
To further assess change in effect sizes based on intervention group, the intercept of the best-fitting LGCM was set at each time point, and intervention group was added as a predictor of each intercept value. For depressive symptoms, there was a gradual increase in effect size over time (4 months post-baseline: β = 0.00, b = −0.04, SE = 0.79, p =.958; 8 months: β = 0.00, b = 0.04, SE = 0.87, p = .964; 12 months: β = 0.01, b = 0.08, SE = 0.89, p = .929; 16 months: β = 0.01, b = 0.06, SE = 0.98, p = .948; 28 months: β = −0.07, b = −0.85, SE = 0.85, p = .317; 40 months: β = −0.13, b = −1.76, SE = 0.94, p = .060). Although effect size never reached statistical significance, it was marginally significant at 3 years post-intervention (p = .060). There were no significant differences in rates of clinically elevated mild or moderate depressive symptoms from baseline (Nmild = 29, 11.0%; Nmoderate = 14, 5.3%) to post-intervention (Nmild = 36, 13.5%; Nmoderate = 17, 6.4%) or 40 months post-baseline (Nmild = 36, 13.5%; Nmoderate = 17, 6.4%), evaluated using the McNemar χ2 test, p = .063 – .607. There were no differences in rates of clinically elevated mild or moderate depressive symptoms based on intervention group at any time point, χ2 (2, N = 201 – 260) = 0.00 – 2.96, p = .085 – 1.00.
Glycemic Control
For HbA1c, only the quadratic-linear piecewise model with a knot at 16 months provided adequate fit across fit indices (see Table 3). Although the quadratic-linear piecewise model fit best, there were no significant changes in HbA1c in the overall model at any time (linear and quadratic slopes, p = .075 – .257). Intervention group did not significantly predict either glycemic control at 40 months post-baseline or change in glycemic control over time.
Blood Glucose Monitoring Frequency
Although a variety of LGCMs assessing blood glucose monitoring frequency were tested, none of the models provided adequate fit (RMSEAs = .09−.13, CFIs = .72−.91, TLIs = .77−.90, SRMRs = .05−.12). To improve LGCM model fit, continuous glucose monitor (CGM) use was added as a time-varying covariate at each of the seven time-points. CGM is a type of diabetes technology that allows the user to track glucose levels without a fingerstick. Many teens report a reduction in frequency of daily blood glucose checks when using CGM (Wong et al., 2014) even though this study was conducted when CGM users were still required to use fingersticks for treatment decision-making. Table 3 displays fit indices of competing models when including CGM use as a covariate. Both the latent-basis model with freed slope-loadings and the quadratic-linear piecewise model with a knot point at 16 months provided adequate fit to the data. Because models were not nested, ΔSBχ2 could not be used to compare their goodness-of-fit. The latent-basis model showed better fit based on AIC, BIC, and SABIC and was therefore selected as most appropriate. There was a statistically significant decline in average daily blood glucose checks across 40 months (slope: b = −0.05, SE = 0.02, p = .007, ß = −0.41), which is associated with an effect size of d = 0.21, and a final model mean of 3.08 checks per day (SE = 0.16, p < .001, ß = 1.63), as compared to baseline of 3.56 checks per day in the model (SE = 0.17, p < .001, ß = 1.92). Intervention group did not significantly predict either blood glucose monitoring frequency at 40 months post-baseline or change in monitoring frequency over time.
Diabetes Management Behaviors4
The linear-linear piecewise model with a knot at 12 months and quadratic-linear piecewise model with a knot at 16 months provided adequate fit to the management behavior data across all fit-indices (see Table 3). These models were not nested, so ΔSBχ2 could not be used to compare them. The quadratic-linear piecewise model fit slightly better than the linear-linear piecewise model based on AIC and SABIC, but not based on BIC. The more parsimonious linear-linear piecewise model with a knot at 12 months was therefore selected as the best-fitting. There was a significant linear decline, with a faster decline from 0 to 12 months (slope1: b = −0.87, SE = 0.35, p = .001, ß = −0.32) than from 12 to 40 months (slope2: b = −0.35, SE = 0.12, p = .004, ß = −0.31), and a final mean rating at 40 months of 60.18 (SE = 1.05, p < .001, ß = 4.15), as compared to a baseline of 65.20 (SE = 0.80, p < .001, ß = 5.76). ). The effect size of change from 0 to 12 months was d = 0.20 and from 12 to 40 month was d = 0.16. Intervention group did not predict either diabetes management behaviors at 40 months post-baseline or change over time.
Time in Range
The linear, quadratic, linear-linear piecewise, and quadratic-linear piecewise models provided adequate fit to the time in range data across fit-indices (see Table 3). The more parsimonious linear model did not fit significantly worse than the quadratic model, ΔSBχ2 (df = 4) = 7.05, p = .133. However, the linear-linear piecewise model fit better than all the other models based on BIC, AIC, and SABIC. Therefore, the linear-linear piecewise model was selected as the most appropriate model. The piecewise model showed stable time in range from 0 to 12 months (slope1: b = −0.12, SE = 0.37, p = .741, ß = −0.04), followed by a decline in time in range from 12 to 40 months (slope2: b = −0.56, SE = 0.18, p = .001, ß = −0.43). The effect size for change from 12 to 40 months was d = 0.22. The model’s baseline mean time in range was 38.27% (SE = 1.06, p < .001, ß = 2.98) and a final mean time in range at 40 months was 33.96% (SE = 1.19, p < .001, ß = 2.72). Intervention group did not significantly predict either time in range at 40 months post-baseline or change in time in range.
Dose Effects
Greater number of sessions attended was associated with less diabetes distress (intercept: b = −0.87, SE = 0.37, p = .018, ß = −0.17), lower HbA1c, (intercept: b = −0.16, SE = 0.04, p < .001, ß = −0.26), and greater blood glucose monitoring frequency (intercept: b = 0.14, SE = 0.04, p = .001, ß = 0.22) at 40 months post-intervention. These effects did not differ by intervention group (diabetes distress: b = 0.57, SE = 0.74, p = .442, ß = 0.15; HbA1c: b = 0.13, SE = 0.08, p = .113, ß = 0.27; blood glucose monitoring frequency: b = −0.03, SE = 0.08, p = .737, ß = −0.06). The number of sessions attended was also associated with change in diabetes management behaviors from 0 to 12 months, b = 0.27, SE = 0.11, p = .010, ß = 0.31, such that when participants attended fewer sessions, diabetes management behaviors declined (b = −2.13, 95% CI [−3.10, −1.15]), but when they attended more sessions, diabetes management behaviors remained stable (b = −0.45, 95% CI [−1.19, 0.29]). This effect did not differ based on intervention group, b = 0.10, SE = 0.21, p = .615, ß = 0.16. There were no other significant dose effects.
Discussion
Results from the three-year outcomes assessment for the STePS multi-site RCT demonstrate the robust effects of PRP T1D in adolescents with T1D. Exposure to both programs was associated with significant declines in diabetes distress during the first-year post-intervention, followed by stabilization in distress over the next two years. Depressive symptoms appeared to remain stable for all participants during the first 16 months post-baseline, followed by a decline in depressive symptoms throughout the three years of follow up assessment. Group differences were also noted, such that participants in the PRP T1D arm reported significantly lower levels of diabetes-specific emotional distress at 3 years post-intervention than those who participated in the EI arm. Moreover, the effect size of this improvement in distress increased over time, reaching statistical significance at two years post-intervention. Similarly, participants in the PRP T1D arm experienced a significant decline in depressive symptoms 16–40 months post-baseline, while participants in the EI arm did not. Although the effect size of the decline in depressive symptoms increased over time, it never reached statistical significance.
The effects observed in the current study are longer-lasting than in prior research (Winkley et al., 2006). A comprehensive review of interventions for reducing diabetes distress in adolescents noted that effects in past studies have typically been short-term, not lasting more than three months post-intervention, even for interventions that included problem solving, cognitive restructuring, and goal setting strategies (Hagger et al., 2016). For example, in the intervention by Serlachius et al. (2016) where participants were assigned to either a CBT-based intervention or usual care, the per-protocol analysis indicated lower distress in the CBT group after three months, but the differences in distress between the intervention groups were no longer statistically significant at 12 months (Serlachius et al., 2016). Our findings differ in that distress declined for all STePS participants in the first year, and the PRP T1D participants’ levels of distress were significantly lower than those in the EI group at three years, with differences between groups growing over time in favor of PRP T1D. Notably, whereas 40% of youth in PRP T1D experienced elevated distress at baseline, only 23% experienced elevated distress 3 years later. Further, 52% of the pooled sample of participants with elevated distress at baseline no longer met criteria for elevated distress at 3 years post-intervention, whereas only 13% of participants without elevated distress at baseline met criteria for elevated distress at 3 years.
The PRP T1D program teaches specific skills designed to improve adaptive coping through relaxation, problem-solving, challenging negative thoughts, and assertiveness strategies. Each of these elements have been identified as helpful for improving mood and psychosocial outcomes for individuals with T1D (Hilliard et al., 2016). It is likely that this skill-training drove the long-term impact on improved mood, above and beyond the effects of meeting in a group with other teens with T1D or other non-specific factors of the diabetes education program (e.g., learning new relevant information, increased support related to shared experiences with other teens, increased salience and focus on diabetes).
HbA1c showed no statistically significant change over the three years of the study regardless of group assignment. There were also no differences and in terms of clinical relevance in change from baseline until 40 months post-baseline, with the mean values of HbA1c changing by less than 0.5%, which is below the cut-off point at which most physicians would consider a change in HbA1c clinically meaningful (Lenters-Westra et al., 2014). Frequency of blood sugar checking and diabetes management behaviors did decline somewhat over the three years (e.g., from 3.6 checks/day to 3.1 checks/day) regardless of group assignment. Time in range initially remained stable through one-year post-intervention, and then declined somewhat regardless of group assignment. The T1D exchange reports increasing HbA1c values and declines in blood sugar monitoring frequency during the adolescent years, with improvements in HbA1c values not seen until emerging adulthood (25 years of age and older; Miller et al., 2015). Other research suggests that teens receiving standard care most often experience worsening glycemic control throughout adolescence (Helgeson et al., 2010; Luyckx & Seiffge-Krenke, 2009). One study found that 85% of teens ages 14 to 18 experience HbA1c increases, and only a minority (under 15%) of teens show stable patterns of glycemic control (Luyckx & Seiffge-Krenke, 2009). The findings from the STePS trial suggest that there may be a protective effect for youth participating in this group-based intervention (regardless of group assignment) by preventing both worsening glycemic control and time in range. It may be that merely meeting together with peers who share the same experiences in living with T1D is, in itself, a therapeutic intervention. Further research is needed to assess that possibility, especially because it may be more feasible for diabetes programs to run group-based education for teens run by nurse CDE’s than psychosocial interventions run by psychologically trained interventionists. In an RCT by Jaser et al. (2014) comparing an online coping intervention with an educational intervention for teens, HbA1c increased in both groups over the first year. Of note, the present study did not include a no-intervention condition that could compare the PRP T1D and EI programs to standard treatment given our interest in offering all participants the opportunity to participate in a program, so further research is needed to test the hypothesis that both programs prevented worsening HbA1c. Nevertheless, national data show rising HbA1c levels during this age span.
Individuals who participated in more intervention sessions reported less distress, lower HbA1c, and better diabetes self-care behaviors at 3 years post-baseline than those who attended less sessions, regardless of group assignment. The impact of the STePS study appears to be related to dose of treatment. Perhaps, as noted above, the more times teenagers met with their peers, the more benefit they received from participation. Alternatively, attending more intervention sessions may simply reflect greater motivation and personal commitment to learning to manage diabetes.
Overall, the sample recruited for this randomized controlled trial aimed at preventing depression and reducing diabetes-specific emotional distress appeared psychologically well adjusted at baseline, with low levels of distress or depressive symptoms. We hypothesize that the STePS intervention may offer even greater benefits to teenagers experiencing elevated levels of depressive symptoms as well as elevated levels of diabetes-specific emotional distress. However, it is also possible that since this was a prevention intervention, the impact of the intervention may not translate to a clinically depressed sample. Future studies, where STePS is offered as a therapeutic intervention instead of as a prevention intervention will assess this possibility.
With respect to diabetes-specific outcomes, at baseline our participants had glycemic control and self-care behaviors that were comparable to the normative population. At the end of three years post-baseline, the teenagers reported decreased diabetes-specific emotional distress, decreased depressive symptoms, and stable glycemic control. Participants in the PRP T1D arm reported greater improvements in distress and depressive symptoms than those in the EI arm. This is likely a result of the specific content of the PRP T1D intervention, which specifically targeted the development of coping and resilience skills.
Given the large sample size and diversity of the participants, our findings are likely to generalize to the larger population of teenagers with T1D. These data demonstrate that a broader implementation of this program, or intervention components, into diabetes clinics may improve psychological outcomes. Further, it may put youth on the road toward less steep rises in HbA1c, which would have both immediate and long-term benefits. While our study had a psychology expert lead the resilience program and a diabetes expert lead the diabetes education program, it may be interesting for future studies to assess the impact of similarly trained interventionists leading both programs.
Limitations include the fact that while we had a racially diverse sample, most participants came from well-educated, two-parent families. It is possible that we did not measure important mediating variables, and we do not know the generalizability of the effects beyond three years. Moreover, analyses of missing data indicate that the data is not missing completely at random for many of the outcome variables. For example, there was more missing data in HbA1c for participants with higher HbA1c at baseline. This suggests that findings should be interpreted with caution as missing data may have affected our findings and therefore our interpretation of patterns of change in HbA1c, or other variables, as well as our conclusions regarding intervention efficacy. Future studies are warranted to determine if there are ways that the intervention could be enhanced to boost its effectiveness.
Public Health Significance:
This study suggests that PRP T1D is an effective treatment for reducing diabetes-specific emotional distress in teenagers with T1D.
This study suggests that PRP T1D is an effective treatment for preventing depressive symptoms in teenagers with T1D.
This study suggests that group-based programs designed for teenagers with T1D may offer a positive psychosocial and metabolic impact regardless of group content
Data Transparency Statement:
We have already published five manuscripts based on this study. The manuscript by Weissberg-Benchell, Rausch, Iturralde, Jedraszko & Hood (2016) entitled: A randomized clinical trial aimed at preventing poor psychosocial lad glycemic outcomes in teens with type 1 diabetes (T1D) reviewed the study protocol and discussed baseline findings. The manuscript by Iturralde, Hood & Weissberg-Benchell (2017) entitled: Avoidant coping and diabetes-related distress: Pathways to adolescents’ type 1 diabetes outcomes assessed the role avoidant coping style and diabetes distress played on health outcomes. The manuscript by Hood, Iturralde, Rausch, & Weissberg-Benchell (2018) entitled: Preventing diabetes distress in adolescents with type 1 diabetes: Results 1 year after participation in the STePS Program assessed the impact of the intervention up to one year post-intervention, on both the psychosocial and metabolic outcomes for the adolescents. The manuscript by Iturralde, Rausch, Weissberg-Benchell & Hood (2019) entitled: Diabetes-related emotional distress over time assessed the impact of the intervention on diabetes distress up to 18 months post intervention, and the fifth manuscript by Shapiro, Vesco, Weil, Evans, Hood & Weissberg-Benchell (2018) entitled: Psychometric properties of the problem areas in diabetes: Teen and parent of teen versions analyzed the psychometric properties of our measure of diabetes-specific emotional distress for teenagers and their parents. This current manuscript assesses a much longer trajectory of findings, assessing outcomes three years post-intervention. The data originate from an NIH-funded, multi-site, longitudinal study aimed at preventing depression and reducing distress in adolescents with type 1 diabetes. Findings reveal that participants experienced a significant reduction in both depressive symptoms and diabetes distress long after the intervention ended.
Acknowledgments
This study was funded by National Institute of Diabetes Digestive and Kidney Diseases (#1R01DK090030)
Korey K. Hood declares investigator-initiated grant support from Dexcom, Inc. The other authors declare they have no conflict of interest related to this research or to this manuscript.
Footnotes
For self-reported measures of diabetes distress, depressive symptoms, and diabetes management behaviors, preliminary analyses assessed longitudinal metric invariance between adjacent time-points using confirmatory factor analysis, to ensure that measures have equivalent meaning over time. The Satorra-Bentler scaled chi-square difference test (ΔSBχ2, Bryant & Satorra, 2012) was used to assess equivalence of factor loadings. If full metric invariance between adjacent time-points was not supported, curve-of-factors models that investigate growth of latent variables over time were used. Curve-of-factors models are similar to LGCMs, but instead of using a composite total score at each time point, a latent variable is modeled, measured by individual items at each time point, which allows for freeing noninvariant parameters (e.g., factor loadings, intercepts, residual variances).
For diabetes distress, metric invariance of a second-order one-factor model with three first-order factors (Shapiro et al., 2018) was supported between adjacent time-points, ΔSBχ2s(15) = 9.80 – 18.21, ps = .252 – .832.
Configural invariance between adjacent time-points was not supported for the one-factor model of depressive symptoms. Accordingly, items with extreme values of skewness (> 3.0) and kurtosis (> 8.0) or ceiling effects (> 70% of participants reporting zero symptoms) were discarded, resulting in an 8-item measure. A partially invariant model provided adequate fit (RMSEA = .04, CFI = .91, TLI = .903, SRMR=.07) with 89% of factor loadings, 91% of intercepts, and 93% of residual variances constrained to be equal across all time-points. Analysis of curve-of-factors models with the partially invariant adapted 8-item measure supported the linear-linear piecewise model with a knot point at 12 months. Intervention group significantly predicted change in depressive symptoms from 12 to 40 months post-baseline (b = −0.01, SE = 0.01, p = .006, β = −0.25), but did not significantly predict change in depressive symptoms from 0 to 12 months (b = 0.01, SE = 0.01, p = .240, β = 0.12) or depressive symptoms at 40 months post-baseline (b = −.06, SE = 0.04, p = .100, β = −0.13).
Configural invariance between adjacent time-points was supported for the one-factor model of diabetes management behaviors (RMSEAs = .04 – .05, CFIs = .91 – .95, TLIs = .90 – .95, SRMRs = .06 – .07), but metric invariance was not, ΔSBχ2s(15) = 26.36 – 36.52, ps = .001 – .034. Follow-up analyses found that configural invariance was not supported across all seven time-points assessed simultaneously. Four items (8, 12, 13, and 14) were subsequently removed from the measure due to low baseline factor loadings (< 0.3). A partially invariant model provided adequate fit (RMSEA = .04, CFI = .91, TLI = .902, SRMR = .07) with 97% of factor loadings, 83% of intercepts, and 92% of residual variances constrained to be equal. Analysis of curve-of-factors models with the partially invariant, adapted 11-item measure supported the linear-linear piecewise model with a knot point at 12 months. Intervention group was not a significant predictor of diabetes management behaviors at 40 months post-baseline (b = 0.08, SE = 0.08, p = .315, β = 0.08) or change in diabetes management behaviors, (linear slope1: b = 0.04, SE = 0.02, p = .069, β = 0.17; linear slope2: b = 0.01, SE = 0.01, p = .345, β = 0.14).
References
- Abualula N, Rodan M, Milligan R, Jacobsen K (2018). Self-rated health among American adolescents with type 1 diabetes in the T1D exchange clinic registry. Journal of Diabetes and its Complications, 32, 83–88. [DOI] [PubMed] [Google Scholar]
- Akaike H (1987). Factor analysis and AIC. Psychometrika, 52(3), 317–332. [Google Scholar]
- Baucom KJW, Queen TL, Wiebe DJ, Turner SL, Wolfe KL, Godbey EI, … Berg CA (2015). Depressive symptoms, daily stress, and adherence in late adolescents with type 1 diabetes. Health Psychology, 34(5), 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA, & Curran PJ (2006). Latent curve models: A structural equation perspective. Hoboken, N.J.: Wiley. [Google Scholar]
- Browne MW, & Cudeck R (1992). Alternative ways of assessing model fit. Sociological Methods & Research, 21(2), 230–258. [Google Scholar]
- Bryant FB, & Satorra A (2012). Principles and practice of scaled difference Chi-square testing. Structural Equation Modeling, 19(3), 372–398. [Google Scholar]
- Chen H, Cohen P, & Chen S (2010). How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics-Simulation and Computation, 39(4), 860–864. [Google Scholar]
- Corathers S, Kichler J, Jones N, & Houchin A, Jolly M, Morwessel N, Crawford P, Dolan L, Hood K (2013). Improving depression screening for adolescents with type 1 diabetes. Pediatrics, 132(5), e1395–e1402. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ, & Willoughby MT (2004). Testing main effects and interactions in latent curve analysis. Psychological Methods, 9(2), 220. [DOI] [PubMed] [Google Scholar]
- de Wit M, Delemarre-van de Waal HA, Bokma JA, Haasnoot K, Houdijk MC, Gemke RJ, & Snoek FJ (2008). Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: A randomized controlled trial. Diabetes Care, 31(8), 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DA, Carcone AI, Slatcher R, Naar-King S, Hains A, Graham A, & Sibinga E (2019). Efficacy of mindfulness-based stress reduction in emerging adults with poorly controlled, type 1 diabetes: A pilot randomized controlled trial. Pediatric Diabetes, 20(2), 226–234. [DOI] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. New York: Guilford. [Google Scholar]
- Feingold A (2009). Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological methods, 14(1), 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham J, Reivich K Jaycox L, & Seligman M (1994). Prevention of depressive symptoms in school children. Behavior Research Therapy. 32 (8), 801–816. [DOI] [PubMed] [Google Scholar]
- Hagger V, Hendrieckx C, Cameron F, Pouwer F, Skinner T, Speight J (2018). Diabetes distress is more strongly associated with HbA1c than depressive symptoms in adolescents with type 1 diabetes: Results from the Diabetes MILES Youth-Australia. Pediatric Diabetes, 19, 840–847. [DOI] [PubMed] [Google Scholar]
- Hagger V, Hendrieckx C, Sturt J, Skinner TC, & Speight J (2016). Diabetes distress among adolescents with type 1 diabetes: A systematic review. Current Diabetes Reports, 16(1), 9. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Snyder PR, Seltman H, Escobar O, Becker D, & Siminerio L (2010). Brief report: Trajectories of glycemic control over early to middle adolescence. Journal of Pediatric Psychology, 35(10), 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard ME, Herzer M, Dolan LM, & Hood KK (2011). Psychological screening in adolescents with type 1 diabetes predicts outcomes one year later. Diabetes Research and Clinical Practice, 94(1), 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard ME, Powell PW, & Anderson BJ (2016). Evidence-based behavioral interventions to promote diabetes management in children, adolescents, and families. American Psychologist, 71(7), 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood KK, Iturralde E, Rausch J, & Weissberg-Benchell J (2018). Preventing diabetes distress in adolescents with type 1 diabetes: Results 1 year after participation in the STePS Program. Diabetes Care, 41(8), 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood KK, Rausch JR, & Dolan LM (2011). Depressive symptoms predict change in glycemic control in adolescents with type 1 diabetes: Rates, magnitude, and moderators of change. Pediatric Diabetes, 12(8), 718–723. [DOI] [PubMed] [Google Scholar]
- Hu L. t., & Bentler PM (1998). Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological methods, 3(4), 424. [Google Scholar]
- Iturralde E, Rauch J, Weissberg-Benchell J, Hood K (2019). Diabetes-related emotional distress over time. Pediatrics, 143(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturralde E, Hood K, & Weissberg-Benchell J (2017). Avoidant coping and diabetes-related distress: Pathways to adolescents’ type 1 diabetes outcomes. Health Psychology, 36(3), 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser SS, Whittemore R, Ambrosino JM, Lindemann E, & Grey M (2008). Mediators of depressive symptoms in children with type 1 diabetes and their mothers. Journal of Pediatric Psychology, 33(5), 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Eiser C, Young V, Brierley S, & Heller S (2013). Prevalence of depression among young people with type 1 diabetes: A systematic review. Diabetes Medicine, 30(2), 199–208. [DOI] [PubMed] [Google Scholar]
- Kovacs M (2003). The Children’s Depression Inventory (CDI): Technical manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Lenters-Westra E, Schindhelm RK, Bilo HJ, Groenier KH, & Slingerland RJ (2014). Differences in interpretation of haemoglobin A1c values among diabetes care professionals. Netherlands Journal of Medicine, 72(9), 462–466. [PubMed] [Google Scholar]
- Lewin AB, LaGreca AM, Geffken GR, Williams LB, Duke DC, Storch EA, & Silverstein JH (2009). Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: The Self-Care Inventory (SCI). Journal of Pediatric Psychology, 34(9), 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx K, & Seiffge-Krenke I (2009). Continuity and change in glycemic control trajectories from adolescence to emerging adulthood: Relationships with family climate and self-concept in type 1 diabetes. Diabetes Care, 32(5), 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, Hau K-T, & Wen Z (2004). In search of golden rules: Comment on hypothesis-testing approaches to setting cutoff values for fit indexes and dangers in overgeneralizing Hu and Bentler’s (1999) Findings. Structural Equation Modeling, 11(3), 320–341. [Google Scholar]
- Matlock KA, Yayah Jones NH, Corathers SD, & Kichler JC (2017). Clinical and psychosocial factors associated with suicidal ideation in adolescents with Type 1 Diabetes. Journal of Adolescent Health, 61(4), 471–477. [DOI] [PubMed] [Google Scholar]
- McGrady ME, & Hood KK (2010). Depressive symptoms in adolescents with type 1 diabetes: associations with longitudinal outcomes. Diabetes Research and Clinical Practice, 88(3), e35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, … Tamborlane, W. V. (2015). Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D Exchange clinic registry. Diabetes Care, 38(6), 971–978. [DOI] [PubMed] [Google Scholar]
- Muthen LK, & Muthen BO (2017). Mplus user’s guide (Eighth ed.). Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- Rao U, & Chen LA (2009). Characteristics, correlates, and outcomes of childhood and adolescent depressive disorders. Dialogues in clinical neuroscience, 11(1), 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reivich KJ, Gillham J, Chaplin TM, & Seligman ME (2005). From helplessness to optimism: The role of resilience in treating and preventing depression in youth. In Brooks G (Ed.), Handbook of resilience in children. New York: Kluwer Academic/Plenum Publishers. [Google Scholar]
- Riley AR, Duke DC, Freeman KA, Hood KK, & Harris MA (2015). Depressive symptoms in a trial behavioral family systems therapy for diabetes: A post hoc analysis of change. Diabetes Care, 38(8), 1435–1440. [DOI] [PubMed] [Google Scholar]
- Schwarz G (1978). Estimating the dimension of a model. Annals of Statistics, 6(2), 461–464. [Google Scholar]
- Sclove SL (1987). Application of model-selection criteria to some problems in multivariate analysis. Psychometrika, 52(3), 333–343. [Google Scholar]
- Serlachius AS, Scratch SE, Northam EA, Frydenberg E, Lee KJ, & Cameron FJ (2016). A randomized controlled trial of cognitive behaviour therapy to improve glycaemic control and psychosocial wellbeing in adolescents with type 1 diabetes. Journal of Health Psychology, 21(6), 1157–1169. [DOI] [PubMed] [Google Scholar]
- Shapiro JB, Vesco AT, Weil LEG, Evans MA, Hood KK, & Weissberg-Benchell J (2018). Psychometric properties of the problem areas in diabetes: Teen and parent of teen versions. Journal of Pediatric Psychology, 43(5), 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg-Benchell J, Rausch J, Iturralde E, Jedraszko A, & Hood K (2016). A randomized clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D). Contemporary Clin Trials, 49, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkley K, Ismail K, Landau S, & Eisler I (2006). Psychological interventions to improve glycaemic control in patients with type 1 diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ: British Medical Journal, 333(7558), 65. [DOI] [PMC free article] [PubMed] [Google Scholar]


