Scheme 2. Synthesis of Pyrimidine Derivatives.
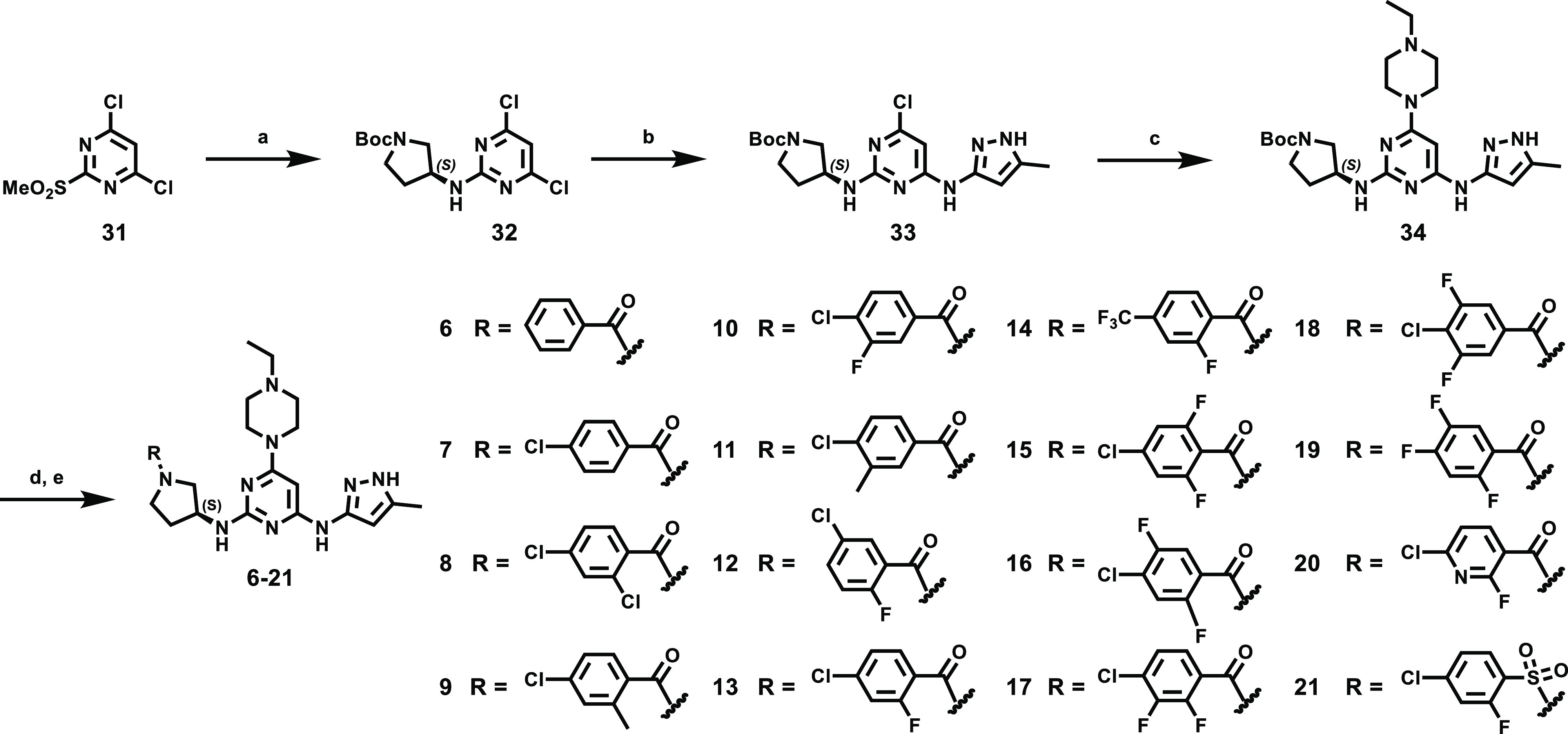
Reagents and conditions: (a) (S)-(−)-1-boc-3-aminopyrrolidine, triethylamine, THF, −70 °C, 6 h, 56%; (b) 3-amino-5-methylpyrazole, NaI, triethylamine, DMSO, 90 °C, 16 h, 85%; (c) 1-ethylpiperazine, triethylamine, 1-pentanol, 140 °C, 2 h, 84%; (d) 2 N HCl in ether, methanol, dichloromethane, 4 h, 99%; (e) various benzoic acid, T3P, triethylamine, DMF, dichloromethane, 16 h, 44–68% or 6-chloro-2-fluoropyridine-3-carboxylic acid, T3P, triethylamine, DMF, dichloromethane, 16 h, 46% (20) or 4-chloro-2-fluorobenzenesulfonyl chloride, triethylamine, dichloromethane, rt, 4 h, 85% (21).
