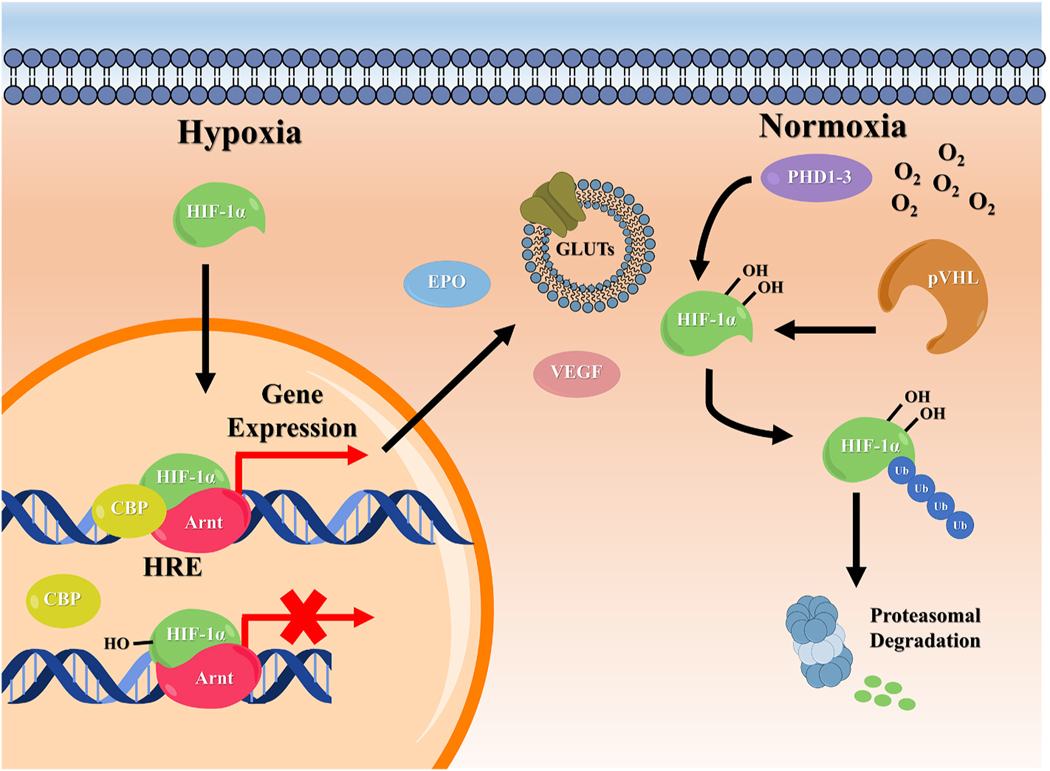
Fig. 1.
Overview of HIF-1α signaling pathways in cells. Local changes in oxygen availability govern the regulation of HIF-1α. During normoxia, PHD enzymes hydroxylate the transcription factor at two proline residues found within the ODDD, leading to ubiquitination and proteasomal degradation. In low oxygen environments (hypoxia), hydroxylation cannot be performed and HIF-1α accumulates and translocates to the nucleus. Here, it dimerizes with the HIF-β unit (Arnt) and binds to the HRE of the target gene promoter. The HIF-1 complex then recruits transcriptional cofactors, such as CBP, resulting in the transcription of target genes such as those governing, EPO, VEGF, and GLUTs production. At moderate oxygen levels, the inhibitory enzyme FIH is also able to hydroxylate HIF-1α at an Asn residue within the C- TAD. This does not affect HIF-1α stability, but prevents the HIF-1 complex from binding to transcriptional coactivators.