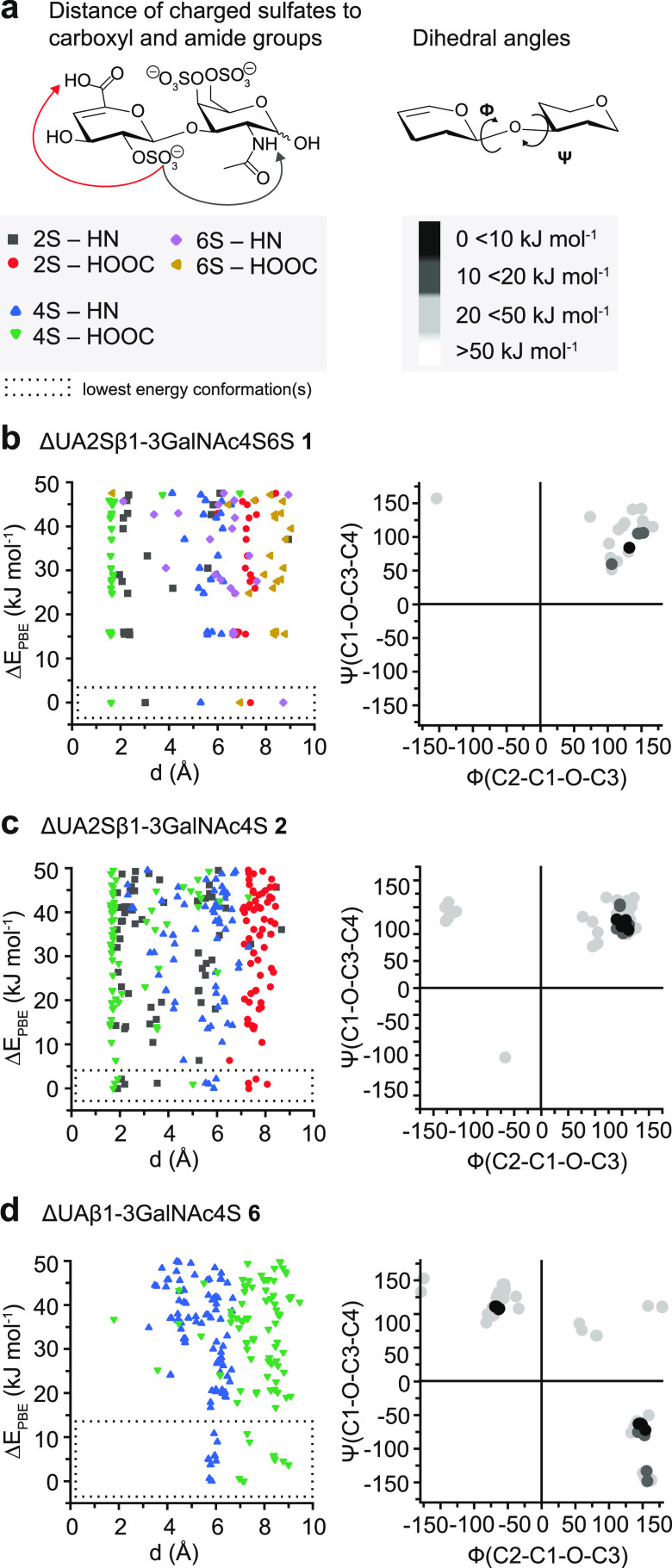
Figure 3.
(a) Left panel: calculation of the intramolecular distance of selected functional groups in Å in conformers with relative energies ΔEPBE < 50 kJ mol–1. Right panel: calculation of the dihedral angles Ψ(C1-O-C3-C4) and Φ(C2-C1-O-C3) in the degree at the glycosidic bond with respect to the relative energies of the conformers. The results are presented in Ramachandran-type plots for glycosidic linkages. (b–d) Results for the triply sulfated disaccharide 1, doubly sulfated disaccharide 2, and singly sulfated disaccharide 6. Generally, an increase in conformational heterogeneity is observed with a decreasing extent of sulfation and charge.