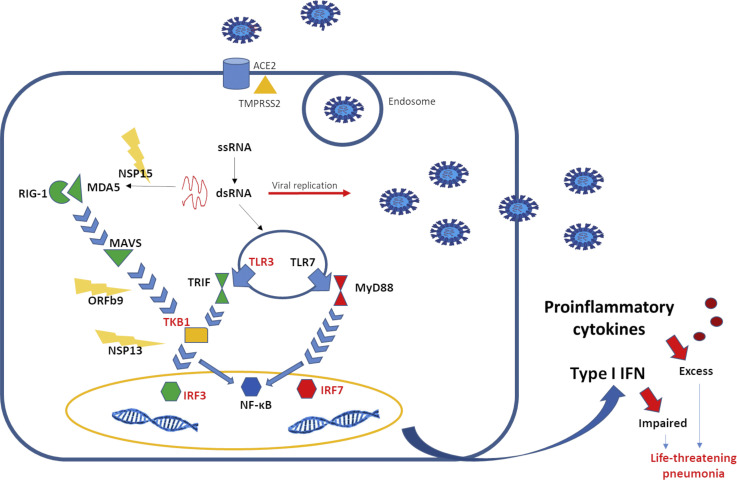
Figure 1.
Virus-host interaction. SARS-CoV-2 protein S binds ACE2 receptor expressed on the surface of alveolar epithelial type 2. After 2 processes of cleavage, which involve the serine protease TMPRSS2, the viral particles are internalized and released into the cytoplasm. As viral replication occurs, double-stranded DNA is recognized by the TLR3 in the endosome and RIG-I and MDA5 in the cytosol. On activation, RIG-I–like receptors and TLRs induce signaling cascades, leading to the activation of transcription factors, such as NF-κB and IRF, and ultimately to the production and release of inflammatory mediators, such as IFN-I and cytokines. SARS-CoV-2 is able to compromise the production of IFN-I, through evasion mechanisms, including NSP15, which cuts the 5' polyuridines, which would have been recognized by MDA5, and ORF9b and NSP13, which interfere with signaling mediated by MAVS and TANK-binding kinase 1, respectively. The importance of a rapid IFN-I–mediated response against the virus is also demonstrated by the fact that mutations in IFN-I–related genes, indicated in red in the figure, are associated with a worse outcome. IRF, Interferon regulatory factor; MAVS, mitochondrial antiviral-signaling protein; MDA5, melanoma differentiation–associated protein 5; NF-κB, nuclear factor kappa B; NSP, Covs nonstructural protein; RIG-I, retinoic acid–inducible gene I; RLR, RIG-I–like receptor; TRIF, TIR-domain–containing adapter-inducing IFN-β; TKB1, TANK-binding kinase 1; TMPRSS2, transmembrane protease serine 2.