Table 2.
Synthesis of various derivatives of 1,2,4-triazoloquinazolinones 5a–j catalyzed by the nano-ordered Melamine@TMG organocatalyst (1).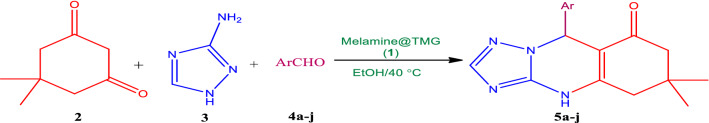
| Entry | Aldehyde 4 | Product 5 | Time (min) | Yielda (%) | Mp (°C) | ||
|---|---|---|---|---|---|---|---|
| Found | Reported | References | |||||
| 1 | PhCHO | 5a | 10 | 98 | 250–252 | 248–250 | 74,77,78 |
| 2 | 2-MeOC6H4CHO | 5b | 20 | 90 | 242–244 | 240–243 | 76,83 |
| 3 | 3,4-(MeO)2C6H3CHO | 5c | 25 | 89 | 225–227 | – | This work |
| 4 | 4-MeC6H4CHO | 5d | 20 | 86 | 262–264 | 264–269 | 74,78 |
| 5 | 4-ClC6H4CHO | 5e | 8 | 98 | 303–305 | 303–305 | 74,78 |
| 6 | 3-O2NC6H4CHO | 5f. | 5 | 97 | 267–269 | 266–269 | 74,78 |
| 7 | 4-O2NC6H4CHO | 5 g | 5 | 98 | 294–297 | 290–294 | 84 |
| 8 | 4-HOC6H4CHO | 5 h | 20 | 91 | 305–308 | > 300 | 74,78 |
| 9 | 3,4-(Cl)2C6H3CHO | 5i | 5 | 99 | 324–326 | – | This work |
| 10 | 4-FC6H4CHO | 5j | 5 | 99 | 257–259 | 258–260 | 85 |
Reaction conditions: dimedone (2, 1 mmol), 3-amino-1,2,4-triazole (3, 1 mmol), aldehyde (4a–j, 1 mmol), Melamine@TMG nanocatalyst (1, 2.5 mol%, 15 mg) in EtOH (2 mL) at 40 °C.
bIsolated yield.
