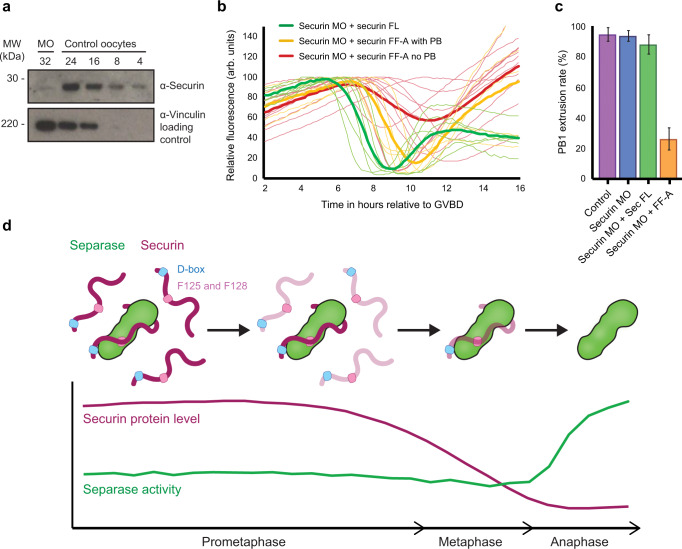
Fig. 4. F125 and F128 are essential for the efficient removal of securin in oocyte meiosis I—where this mechanism is perturbed, oocytes fail to divide.
a Quantification of securin morpholino knockdown. Western blot of control and securin morpholino (MO) injected oocytes collected 5.5 hours post GVBD (numbers of oocytes loaded per lane are indicated). Quantification of protein bands indicates an 87% knockdown of securin in MO-injected oocytes. The lower blot was probed for vinculin and used as a loading control. b Securin protein levels were knocked down by morpholino (MO) injection. Oocytes were subsequently injected with either securin FL (green traces, n = 6) or securin FF-A (yellow and red traces, n = 15). Although securin FL-injected oocytes had normal rates and timings of PB1 extrusion, FF-A-injected oocytes were either delayed (yellow traces) or blocked in MI (red traces). Destruction profiles are relative to GVBD. c Quantification of PB1 extrusion rate between control oocytes (purple column, n = 50), securin MO-injected oocytes (blue column, n = 29), and securin MO-injected oocytes subsequently injected with either securin FL (green column, n = 15) or securin FF-A (orange column, n = 23). All n numbers refer to the number of individual oocytes analysed over a minimum of three independent experiments. Data presented as mean values with error bars ± SEM. d A model summarising the mechanism by which a pool of excess non-separase-bound securin is preferentially targeted for destruction in late prometaphase I in oocytes. This mechanism involves both the D-box and a second region within the SIS of securin where F125 and F128 are essential. Following this, a smaller pool of separase-bound securin becomes a destruction target in metaphase.