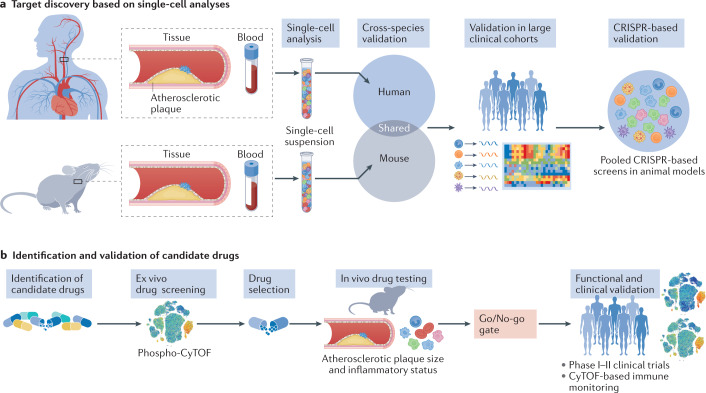
Fig. 3. Integration of single-cell methods for the discovery and validation of drug targets.
a | Single-cell studies in humans and mice provide information about the disease. Whereas studies in humans define the actual disease state, mechanistic studies in mice can aid in the understanding of how perturbations affect the disease. Integration and cross-species validation of these studies can be used to identify novel molecular targets. Understanding these molecular pathways in large clinical cohorts can be used as validation and then secondarily validated in animal models with the use of pooled CRISPR screening. b | When new targets are identified and validated, candidate drugs can be assessed for their specific effect in modulating these pathways. One evaluation method is phosphoproteomics with cytometry by time of flight (phospho-CyTOF). In vivo testing in animals can be used to investigate further the efficacy of the drug and to evaluate the go/no-go decisions to enter clinical phases of drug development. The adoption of immune monitoring in the early phases of clinical trials can provide crucial information on patient selection and efficacy for the design of future end point-driven clinical trials.