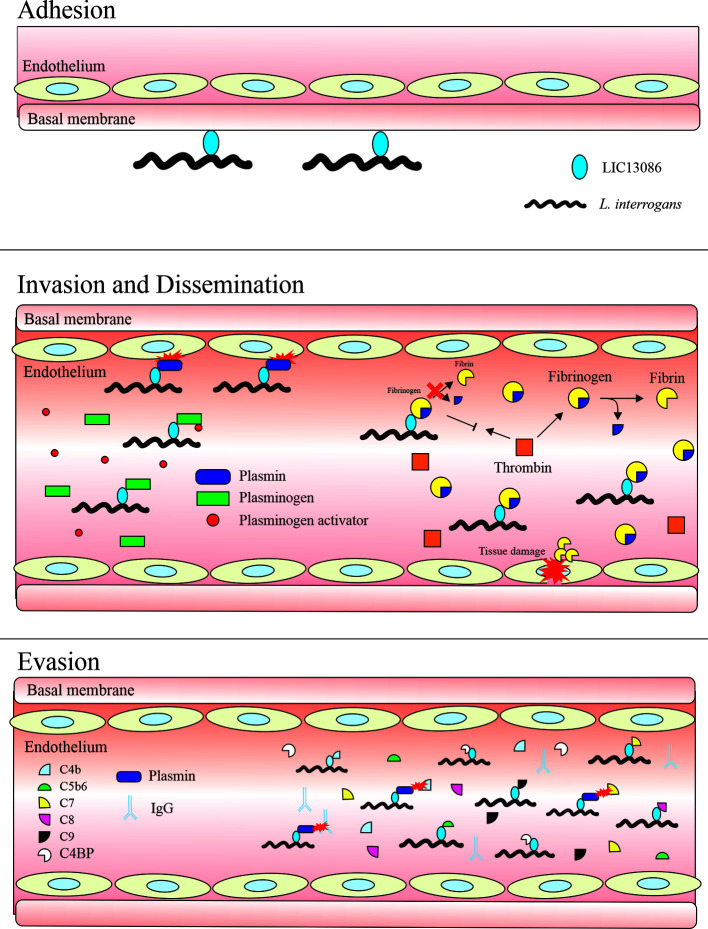
Figure 10.
Schematic model representing the possible roles of the protein LIC13086 in Leptospira–host interactions and pathogenesis. LIC13086 is a putative lipoprotein experimentally shown to be located in the leptospiral outer membrane. By binding to ECM and basal membrane components, namely laminin, LIC13086 can participate in the adhesion process of leptospires to the host tissues. The binding of LIC13086 to plasminogen/plasmin can contribute to bacterial dissemination, invasion, and immune evasion, through various mechanisms. The plasminogen is first recruited to the bacterial surface via bacterial receptors, including LIC13086. The leptospiral-bound plasminogen is converted into plasmin by host-derived plasminogen activators, such as uPA. Plasmin is able to degrade ECM and basal membrane components, resulting in enhanced bacterial penetration and tissue damage. Plasmin also cleaves immunoglobulins and complement molecules, thus favoring immune evasion. By binding to fibrinogen, LIC13086 inhibits its cleavage by thrombin, thus reducing fibrin formation, which can have a consequence in the loss of hemostasis. The complement system comprises various components and regulators. By sequestering the host complement proteins and regulators through various surface proteins, including LIC13086, leptospires can inhibit the complement deposition on the outer membrane, thus avoiding the membrane attack complement (MAC) assembly.