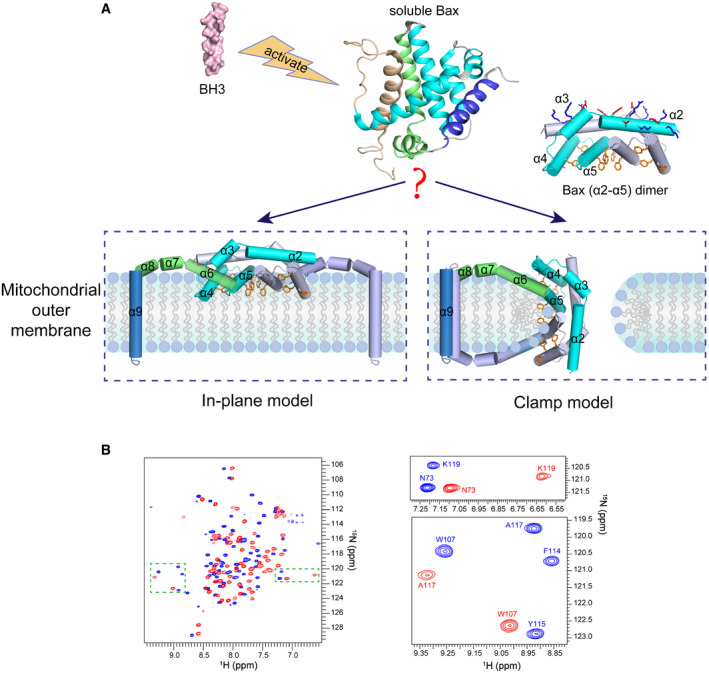
Figure 1. Models for the membrane topology of Bax and NMR spectra of Bax (α2–α5).
-
AThe “in‐plane” and the “clamp” models for the membrane topology of pore‐forming Bax protein. After activation by BH3 proteins, the soluble Bax protein unfolds the α‐helical bundle structure and inserts the α9 helix into the MOM. The tail‐anchored Bax proteins dimerize via their α2, α3, α4, and α5 helices resulting in an amphipathic structure (shown as Bax (α2–α5) dimer with the protomers in cyan or gray color) with a polar surface containing positive and negative charged α2 and α3 residues (blue and red sticks) and a nonpolar surface containing aromatic α4 and α5 residues (orange rings). According to the in‐plane model, the α2–α5 dimer engages the planer cytosolic surface of the MOM with the aromatic α4 and α5 residues projected into the cytosolic leaflet of the lipid bilayer to generate membrane tension thereby inducing a lipid pore with radially arranged lipids at the rim. In contrast, the clamp model positions the α2–α5 dimer at the lipid pore rim with the aromatic α4 and α5 residues intercalated between the radiated lipids to reduce line tension thereby stabilizing the pore. As illustrated, the membrane topologies of other helices are also different between the models. The structures of the soluble Bax protein and the α2–α5 dimer were generated from PDB files 1F16 and 4BDU, respectively, using PyMOL program. The structures of other helices are modeled based on the corresponding structures in the soluble Bax protein.
-
B2D 1H–15N TROSY‐HSQC spectra of DMPC/DHPC bicelle‐bound Bax (α2–α5) (blue) and soluble Bax (α2–α5) (red) at 600 MHz. The magnified regions highlight the spectral changes for some residues in the bicelle‐bound and soluble proteins.
