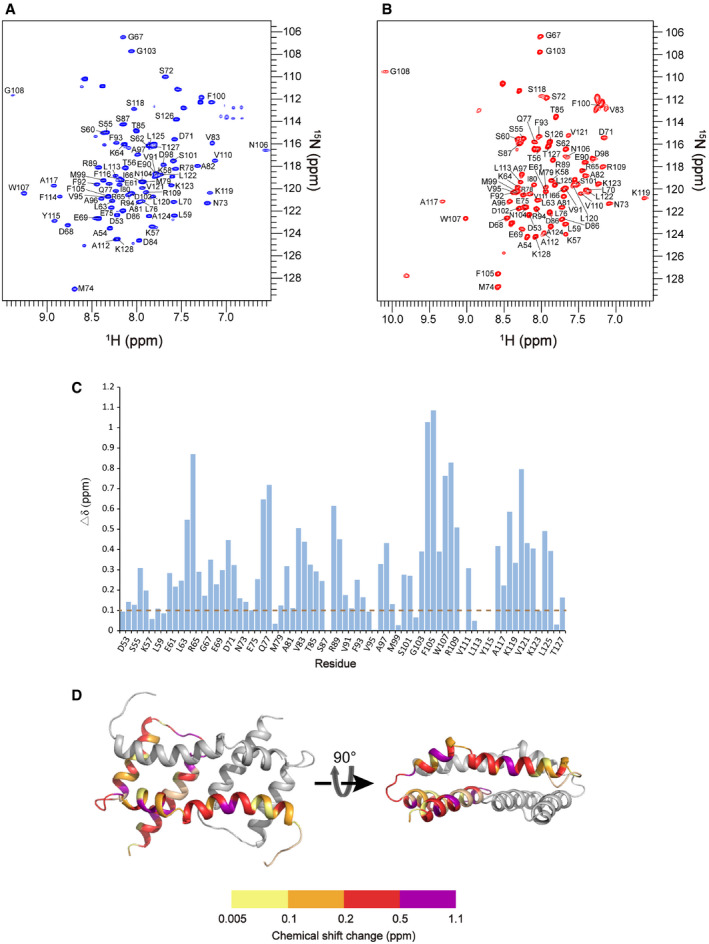
Figure EV1. Related to Figs 1 and 2. NMR spectra of Bax (α2–α5) in bicelles and solution with assignments.
-
A2D 1H–15N TROSY‐HSQC spectrum of the uniformly [15N, 13C, 2H]‐labeled Bax (α2–α5) protein in DMPC/DHPC bicelles (q = 0.5) recorded at 1H frequency of 600 MHz. The backbone amide resonances were assigned to the indicated residues.
-
B2D 1H–15N TROSY‐HSQC spectrum of the uniformly [15N, 13C, 2H]‐labeled soluble Bax (α2–α5) protein recorded at 1H frequency of 600 MHz. The backbone amide resonances were assigned to the indicated residues.
-
CNMR chemical shift changes of Bax (α2–α5) upon the addition of the bicelles. The plot of chemical shift changes between the spectra of bicelle‐bound Bax (α2–α5) and soluble Bax (α2–α5). The combined chemical shift changes (Δδ) were calculated by using the equation: where ∆δH and ∆δN are chemical shift changes (in ppm) in the 1H and 15N dimensions, respectively, and ω H and ω N are normalization factors (ω H = 1.00, ω N = 0.15).
-
DThe chemical shift changes of Bax (α2–α5) were mapped to the structure of the bicelle‐bound Bax (α2–α5), colored in one chain according to the scale of chemical shift changes shown below.
Source data are available online for this figure.
