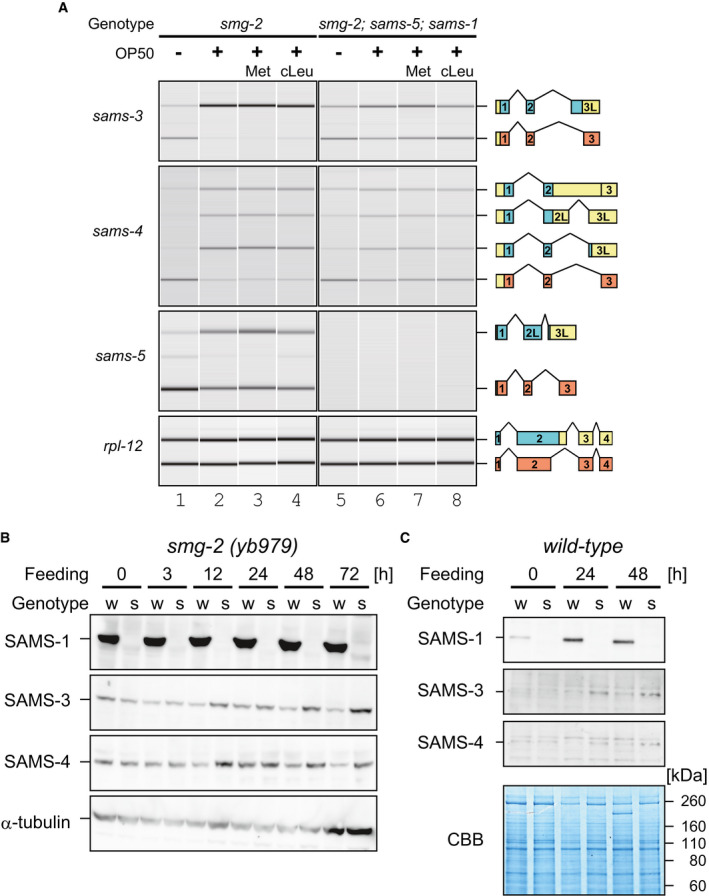
Western blot analysis of SAMS‐1, SAMS‐3, and SAMS‐4 during larval development in the
smg‐2
(yb979) (B) and wild‐type (C) backgrounds. Genotypes of the worms are
smg‐2 (w) and
smg‐2; sams‐5; sams‐1 (s) in (B) and wild‐type (w) and
sams‐1 (s) in (C). Synchronized L1 larvae of each strain were incubated with OP50 at 20°C and subjected to Western blot analysis at indicated time points. Anti‐β‐tubulin (B) or Coomassie Brilliant Blue (CBB) staining (C) was used as a loading control. Specificity of the antibodies is confirmed in Appendix␣Fig
S12. Note that upregulation of SAMS‐1 protein in the wild type during larval development in (C) is consistent with feeding‐induced upregulation of
sams‐1 mRNA in Fig
EV3D.