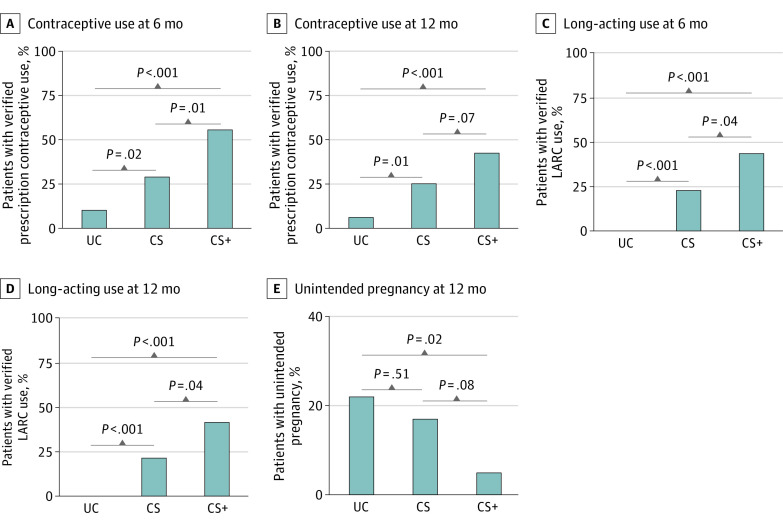
Figure 2. Percentage of Participants With Verified Prescription Contraceptive Use, Verified Long-acting Reversible Contraceptive Use, and Who Experienced an Unintended Pregnancy.
A and B, The percentage of participants with verified prescription contraceptive use at the end of the 6-month intervention period and at the end of the 12-month trial in the 3 conditions. Verified use was defined as a self-report of adherence to a method for the 28 days leading up to the assessment and verification based on pill count (pill), medical record review (injection), palpation (implant), or pelvic examination (ring, intrauterine device [IUD]). C and D, Percentage of participants with verified long-acting reversible contraceptive (LARC; IUD or implant) use at the end of the 6-month intervention period and at the end of the 12-month trial. E, Percentage of participants who experienced an unintended pregnancy during the 12-month trial period. For panels A-D, n = 48 for usual care (UC), n = 48 for contraceptive services (CS), and n = 42 for contraceptive services plus incentives (CS+) and for panel E, n = 45 for UC, n = 42 for CS, and n = 41 for CS+. All Cochran-Armitage χ2 tests for trend were significant (P ≤ .025). Significance levels for pairwise comparisons are based on Pearson χ2 tests.