Abstract
Objective
This study explored how women’s beliefs about drug safety and interactions with their health care providers influenced their decisions to continue arthritis medications during pregnancy and lactation.
Methods
We collaborated with ArthritisPower, a patient‐powered research network, and CreakyJoints, its partner online community, to develop and disseminate a survey among members with inflammatory arthritis who had at least one pregnancy after diagnosis. Participants’ free‐text responses were evaluated by using thematic analysis.
Results
Women in the sample were 40 years old on average (N = 66). Nineteen of their pregnancies had ended in fetal loss. Fifteen percent of all pregnancies were exposed to methotrexate. Among women who used safe arthritis medications, up to 80% discontinued treatment either in preparation for pregnancy or during pregnancy or lactation. Women’s decisions to continue medications during pregnancy were influenced by their perceptions of safety and advisement from health care providers, although they often described that advice about medication safety was inconsistent between providers.
Conclusion
Women often chose to endure active inflammatory arthritis rather than to use disease‐modifying antirheumatic drugs because of concerns about medication safety during pregnancy and lactation. Conflicting medical advice from health care providers undermined patients’ trust in their providers and in the safety of their medications. The high rate of peripartum exposure to methotrexate, a fetotoxic drug, underscores the need for better family planning care for women with childbearing potential.
INTRODUCTION
Studies indicate that 31% to 62% of women with inflammatory arthritis (eg, rheumatoid arthritis [RA], spondyloarthritis [SpA], and juvenile idiopathic arthritis [JIA]) discontinue disease‐modifying antirheumatic drugs (DMARDs) while pregnant or breastfeeding (1, 2, 3). In some cases, this decision may be appropriate: DMARDs with established fetotoxicity should be discontinued before conception, and RA disease activity has been found to improve during some pregnancies, negating the need for medical therapy (4, 5, 6).
However, other women who discontinue medications in the context of pregnancy or breastfeeding may face considerable health consequences. Poorly controlled inflammatory arthritis among mothers with RA and JIA has been associated with adverse fetal outcomes, including prematurity and low birth weight (7, 8, 9, 10). Active arthritis has been associated with subfertility among women with RA (11, 12). Arthritis flares during pregnancy or post partum may impair a woman’s physical functioning, her quality of life, and her ability to rear her children (5, 13).
Because medications that are safe and compatible with both pregnancy and lactation exist (14, 15, 16, 17), absolute medication discontinuation is usually not a requirement but rather a choice for many female patients. We partnered with a patient‐powered research community to create and disseminate a reproductive health survey exploring how women with inflammatory arthritis gather information about medication risks, weigh the risks and benefits of treatment, and ultimately decide to continue or discontinue their treatments while pregnant or breastfeeding.
PATIENTS AND METHODS
Study population
Our study population consisted of patients who were registered members of ArthritisPower, a patient‐powered registry, and CreakyJoints, an online community of individuals with RA, JIA, SpA, systemic lupus erythematosus (SLE)–related arthritis, and inflammatory bowel disease–related arthritis, among other forms of arthritis. Recruitment emails were sent widely to the members of ArthritisPower and CreakyJoints between March and June 2017. Emails described the study and the inclusion criteria, which included a diagnosis of inflammatory arthritis and age between 18 and 50 years. Emails also included a link to the survey and consent documentation. We were unable to restrict the email solicitations by sex or age, and CreakyJoints does not gather information about members’ ages; therefore, some emails were sent to members who were ineligible to participate in the study. We also could not verify if member’s email addresses were active or if recruitment emails were flagged as spam by email servers.
Because the current analysis focused on people with childbearing potential, the following enrollment data reflect female members of Arthritis Power and CreakyJoints (n = 13,484). Among these women, 25.6% opened at least one of three email solicitations about the study (n = 3451). A total of 13.8% selected the survey link (n = 476). The survey software automatically excluded individuals who did not meet age criteria or did not provide consent. Participants with incomplete or, in one case, duplicate survey data were excluded. A total of 267 women met eligibility criteria. Because the current analysis focused on medication decision‐making during pregnancy and lactation, an additional inclusion criterion of this study was that respondents must have experienced a pregnancy after their arthritis was diagnosed; 66 of the 267 women (24.7%) met this criterion. To ensure that their responses reflected only pregnancies that occurred after the diagnosis of inflammatory arthritis, women were prompted to share information only about their most recent pregnancy.
Survey content
ArthritisPower patient governors presented reproductive health questions from members to the study team; together, we created a comprehensive survey consisting of a total of 183 questions about pregnancy, fertility, breastfeeding, lactation, and contraception; approximately 75% of the questions were presented in multiple‐choice format, and the remaining questions were written in free‐text format. An example free‐text question was “Did your doctors have different opinions about what arthritis medications you could take during pregnancy?” The survey design incorporated branching logic so that respondents were presented with questions that were most relevant to their experiences. The current study focused on responses related to women’s decision‐making and information‐seeking regarding arthritis medication use during pregnancy and lactation. Additional details about our survey have been previously described (18). This study was approved by the Duke University Institutional Review Board (PRO00079454).
Analysis
Our survey format facilitated the collection of quantitative and qualitative data. Our quantitative analysis was generally descriptive because we intended to explore women’s experiences rather than to test hypotheses. However, several key articles were published around 2011 and 2012 that suggested that hydroxychloroquine (HCQ) and tumor necrosis factor α inhibitors (TNFis) were safe to use through pregnancy and lactation (19, 20, 21, 22, 23, 24). Because these data may have changed how clinicians counseled about medication risk, Fisher’s exact tests were used to assess whether medication use and physician’s recommendations for medication use through prepregnancy, pregnancy, and lactation changed by the year in which women reported their last pregnancy. In addition to this exploratory analysis, we used Fisher’s exact tests to assess the extent to which women’s perceptions about a medication’s safety during pregnancy were associated with their continuation of the medication during pregnancy. The 2020 American College of Rheumatology (ACR) reproductive health guideline was used to categorize medications by their safety profiles during pregnancy and lactation (17).
Survey responses are presented as frequencies, means, and SDs. Analyses were conducted by using SAS 9.4 (SAS Institute, Inc.).
Thematic analysis was used to organize and analyze free‐text comments. We selected a deductive approach because the objectives of this study had been identified in advance and provided a framework for the analysis (25, 26). The qualitative analysis was conducted by MBT, a rheumatologist and women’s health specialist with formal qualitative training, in coordination with MEBC, a rheumatologist with expertise in the reproductive health of women with rheumatic diseases, and AME, an investigator in rheumatology and women’s health who had developed the survey. We first familiarized ourselves with the raw data by reading the free‐text responses and identified a thematic framework on the basis of the study objectives. We indexed the data by applying the framework to each of the transcripts and reorganized the data on the basis of the relevant themes. The thematic analysis was shared with the study team as a form of analyst triangulation and to ensure that the major themes were clearly positioned within the context of the study objectives. These themes are presented as subheadings in the Results section. We also present individual free‐text responses that were particularly representative of a specific theme and were clearly written.
RESULTS
Table 1 presents the patients’ demographic characteristics (N = 66). Most women were White (77%), were college educated (68%), and had RA (89%), but patients with SpA, JIA, and SLE were also represented in the sample. The average age at the time of arthritis diagnosis was 22.2 years old, and the average age at the time of survey completion was 40.3 years. Most women indicated current or historical use of at least one DMARD or prednisone (95%). Women had an average of 1.7 pregnancies after their disease diagnosis (SD 1.0), with a range of one to five pregnancies overall. On average, 8.3 years (SD 6.8) had elapsed between their last pregnancy and the completion of the survey. Forty‐seven pregnancies were viable, whereas 19 pregnancies ended in fetal loss. Our survey forms did not elicit additional information about these losses (eg, trimester or cause of death). Because only one woman in the sample used a non‐TNFi biologic drug around the time of pregnancy/lactation, and because relatively few safety data are currently available for newer drugs (15, 17), questions about biologic DMARDs focused on TNFis.
Table 1.
Patient characteristics
| Demographics | Value |
|---|---|
| Current age, mean (SD), years | 40.3 (6.2) |
| Age at diagnosis, mean (SD), years | 22.2 (10.2) |
| Race, n (%) | |
| White | 51 (77) |
| Black, Asian, Native American, Multi‐racial, Race not specified | 15 (23) |
| Education, n (%) | |
| College degree or higher | 45 (68) |
| Less than college degree | 21 (32) |
| Type of arthritis (could identify ≥1), n (%) | |
| Rheumatoid arthritis | 59 (89) |
| Spondyloarthritis | 12 (18) |
| Juvenile idiopathic arthritis | 9 (14) |
| Systemic lupus erythematosus | 3 (5) |
| Medications ever used, n (%) | |
| Prednisone | 60 (91) |
| TNF‐α inhibitor | 54 (82) |
| Hydroxychloroquine | 46 (70) |
| Methotrexate | 55 (83) |
| Sulfasalazine | 26 (39) |
| Leflunomide | 20 (30) |
| Other biologic | 28 (42) |
| NSAIDs | 63 (95) |
| None | 0 |
| Number of pregnancies, total, mean (SD) | 2.5 (1.6) |
| Number of pregnancies, after diagnosis, mean (SD) | 1.7 (1.0) |
Abbreviations: NSAID, nonsteroidal anti‐inflammatory drug; TNF‐α, tumor necrosis factor α.
Our exploratory analysis suggested that peripartum use of TNFis and HCQ increased after the year 2012. Among women prescribed TNFis, 18% of women continued these medications through pregnancy post‐2012, whereas only 5% of women continued these medications pre‐2012 (P = 0.006). HCQ also seemed to be used more frequently during pregnancies post‐ versus pre‐2012 (18% vs 5%), although this comparison did not reach statistical significance.
Theme 1: patients worry about the safety of their traditional or biologic DMARDs during pregnancy and lactation
A central theme that emerged from surveys and free‐text responses was that respondents were worried about the safety of their traditional or biologic DMARDs during pregnancy and lactation. Table 2 presents patients’ perceptions of medication safety during pregnancy versus expert opinion. Forty percent of women felt that no medications were safe to use in pregnancy; one woman explained, “I feel none [medication] should be used.” Other women selected prednisone (41%), TNFis (15%), nonsteroidal anti‐inflammatory drugs (NSAIDs) (11%), HCQ (9%), and acetaminophen (2%) as safe medications. No women selected sulfasalazine, a pregnancy‐ and lactation‐compatible DMARD, as being compatible with pregnancy (17). No women also selected methotrexate or leflunomide as being compatible with pregnancy; both of these medications have teratogenic potential (17).
Table 2.
Patients’ perceptions of medication safety in pregnancy versus expert opinion
| Patient perceives as safe, n (%) | Experts’ perceptions of safety | ||
|---|---|---|---|
| Overall (N = 66) | Among women who had ever used the medication | ||
| Prednisone | 24 (36) | 24/60 (40) | Safe |
| Hydroxychloroquine | 6 (9) | 5/46 (11) | Safe |
| Sulfasalazine | 0 (0) | 0/26 (0) | Safe |
| NSAIDs (nonselective) | 7 (11) | 7/63 (11) | Safe |
| TNF‐α inhibitor | 10 (15) | 10/54 (19) | Safe |
| Methotrexate | 0 (0) | 0/55 (0) | Not safe |
| Leflunomide | 0 (0) | 0/20 (0) | Not safe |
| Other biologic/small molecule | 0 (0) | 0/28 (0) | Unclear safety profile |
| None of the above | 27 (41) | 27/66 (41) | n/a |
Patients were allowed to select more than one response. Expert opinion was abstracted from the American College of Rheumatology reproductive health guideline (17).
Abbreviations: n/a, not applicable; NSAID, nonsteroidal anti‐inflammatory drug; TNF‐α, tumor necrosis factor α.
Theme 2: women’s decisions to continue medications during pregnancy were influenced by their perceptions of safety and by advisement of health care providers
In this sample, up to 80% of women discontinued either a nonfetotoxic DMARD, prednisone, or NSAIDs immediately prior to or during pregnancy (Figure 1). No women used sulfasalazine or leflunomide during or immediately prior to pregnancy. Medication usage in the sample is detailed below.
Figure 1.
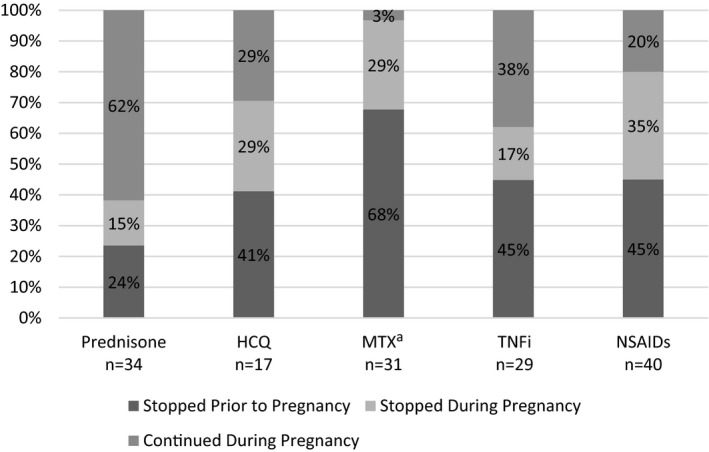
Associations between medication continuation, medication discontinuation, and pregnancy in the sample. a One patient reported taking MTX in pregnancy and had a pregnancy loss; she did not report stopping the MTX. HCQ, hydroxychloroquine; MTX, methotrexate; NSAID, nonsteroidal anti‐inflammatory drug; TNFi, tumor necrosis factor α inhibitor.
NSAIDs
NSAIDs are conditionally recommended during pregnancy, except during the third trimester or if the patient is experiencing difficulty in conceiving a pregnancy (17). Thirty‐two women discontinued NSAIDs in preparation for or during pregnancy, whereas eight women continued NSAIDs throughout pregnancy. Three women discontinued NSAIDs because their arthritis improved. Women who believed that NSAIDs were safe to use during pregnancy were significantly more likely to use NSAIDs during pregnancy (43% vs 8%; P = 0.03). Some women received conflicting opinions about NSAID safety from different physicians (13%). One patient described, “My rheumatologist told me it was safe to use NSAIDs while trying to conceive. My [obstetrician] told me that NSAID use in early pregnancy has a high risk of spontaneous abortion.” This contrasted with another woman’s experience: “Rheumatologist and high‐risk obstetrician had different opinions on when to stop [NSAID]. Rheumatologist said right away, obstetrician said 27 weeks.”
HCQ
HCQ is safe to use during pregnancy (17). Twelve women discontinued HCQ in preparation for or during pregnancy, whereas five women continued HCQ through pregnancy. Women who believed that HCQ was safe to use during pregnancy were more likely to use HCQ during pregnancy than women who felt it was unsafe (67% vs 2%; P = 0.0001). Most women were instructed to discontinue HCQ by a physician (75%). Few women shared any vignettes about their experiences with HCQ, although one woman wrote, “I only took it because I didn’t know I was pregnant.”
Prednisone
Prednisone is conditionally recommended to use during pregnancy, and doses lower than 20 mg daily are favored (17). Thirteen women stopped prednisone in preparation for or during pregnancy, whereas 21 women continued prednisone. Women who believed prednisone was safe to use during pregnancy were significantly more likely to continue it during pregnancy (71% vs 10%; P < 0.0001). Half of women discontinued prednisone on the basis of advice from their physicians (54%), whereas 23% were told that prednisone was compatible with pregnancy but chose to discontinue. Several women described that they tried to limit usage during pregnancy; one participant described, “I took less and some days didn’t need it at all.”
TNFis
TNFis are safe to use during pregnancy (17). Twenty‐two women discontinued TNFis in preparation for or during pregnancy, and seven women continued TNFis through pregnancy. Women who believed that TNFis were safe versus unsafe to use during pregnancy were more likely to use TNFis during pregnancy (50% vs 4%; P = 0.0005). Two women stopped TNFis because their arthritis improved. Other women discontinued TNFis because of physician advice (68%); 14% of women stopped TNFis because of lack of consensus between providers. One woman described, “Nobody would commit to a 100% yes to stay on [TNFi] until I saw my perinatologist.” Another woman who used a TNFi during pregnancy wrote as follows:
My doctor and I decided I would stop [TNFi] as soon as I found out I was pregnant to see if I would achieve remission while pregnant. I was off for most of my pregnancy…but my inflammation got to the point where my doctor and I decided it was a bigger risk to me and the baby than [TNFi] was.
Methotrexate
In contrast to HCQ, prednisone, NSAIDs, and TNFis, methotrexate is a fetotoxic DMARD and abortifacient drug that should have been discontinued prior to pregnancy for all women (15, 16). Thirty‐one women used methotrexate before pregnancy. However, whereas 70% stopped methotrexate in preparation for pregnancy, 30% of women became pregnant while using methotrexate. All women were counseled by their physicians to discontinue methotrexate, with exception of one woman who had experienced an unintended pregnancy while using methotrexate. This pregnancy ended in fetal loss, as she explained: “Methotrexate caused abortion…pregnancy was not planned—it was an oops.” No other women who became pregnant while using methotrexate shared free‐text responses about their experiences or their pregnancy outcomes.
Theme 3: women generally did not believe that arthritis medications were compatible with breastfeeding
Seventy‐nine percent of women who had a viable pregnancy breastfed for an average of seven months (range: <4 weeks to 29 months). Most women tried to avoid using DMARDs or prednisone while breastfeeding (78%). Five of ten mothers did not breastfeed at all; although none were using a fetotoxic DMARD, they decided not to breastfeed to avoid exposing their infants to any medications.
Medications used by breastfeeding mothers included prednisone (46%), NSAIDs (30%), TNFis (14%), and HCQ (5%) (Figure 2). No women used methotrexate or leflunomide while breastfeeding. One‐third of breastfeeding mothers did not use any DMARDs, NSAIDs, or steroids while breastfeeding. Although no breastfeeding mothers used a TNFi pre‐2012, five women used a TNFi post‐2012. Of the 11 women who used NSAIDs while breastfeeding, NSAIDs were used more frequently post‐ versus pre‐2012 (44% vs 16%; P > 0.05); otherwise, there were no temporal trends in medication use.
Figure 2.
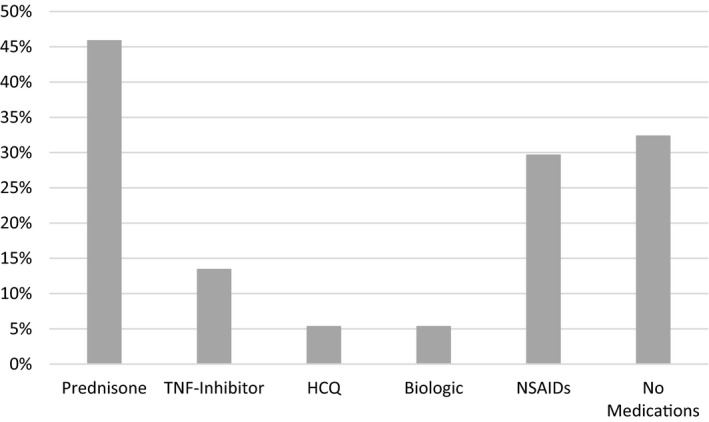
Medication use among lactating women (n = 37). HCQ, hydroxychloroquine; NSAID, nonsteroidal anti‐inflammatory drug; TNF, tumor necrosis factor α.
Among women who breastfed, one‐third described that breastfeeding was physically challenging because of arthritis pain. However, most of these women did not believe that breastfeeding was compatible with use of any medication in multiple‐choice and free‐text responses. Several women expressed that their priority was to eliminate medication exposure to their infants, even at the expense of their physical functioning: “For the first 6 months I flared while breast feeding…I would have my spouse hold the baby up to my breasts (to) breast feed. I was in bed for 6 months.” More than half of women (54%) stopped breastfeeding because their disease activity became too severe to continue to withhold treatment: “I breastfed as long as I could using medications that were considered moderately safe, but when it got to the point that my RA was so bad that I could barely hold my baby, I knew I needed stronger RA medications and I reluctantly weaned.” Another woman expressed, “I decided that it would be better for my baby to have a mother who was able to hold her than to breastfeed.” Among women who stopped breastfeeding to restart treatment, none had been using a fetotoxic DMARD before or during pregnancy and only one woman had been prescribed a non‐TNFi biologic DMARD.
Theme 4: health care providers often offered conflicting advisement about medication safety in pregnancy and lactation
Women spoke with their rheumatologists (80%), obstetricians (73%), and/or primary care providers (PCPs) (33%) about the safety of their antirheumatic drugs during pregnancy. Most conversations occurred before pregnancy (74%), although 9% of women never talked about medications with their providers at any point before, during, or after pregnancy. Patients felt more confident in the medication recommendations they received from maternal‐fetal medicine specialists (76%) than from their rheumatologists (57%), obstetricians (38%), or PCPs (14%). In approximately half of cases in which a woman stopped a medication during pregnancy, at least one provider had advised her to do so.
Overall, 24% of women in our sample reported that their health care providers had differing opinions about the safety of their medications during pregnancy; however, this appeared to vary by the time frame in which they had their children. Women who had children before 2012 reported that medication risk counseling was consistent between health care providers (94%), whereas half of women who had pregnancies after 2012 reported discrepancies between their providers’ recommendations (P = 0.0002). Most women described that inconsistent medical advice undermined their decision‐making and trust in their providers’ advice and expertise. One woman described, “One (health care provider) didn’t want me to take prednisone at all, the other was okay with a very small daily dose. Both were okay with opioids, but my [PCP] said no opioids at all. Making my decisions very hard.” One woman expressed tensions regarding her providers’ advice and expertise: “Obstetrician was not clear enough on medication I could take, my [rheumatologist] was against the pregnancy, but was more knowledgeable, my PCP was hands off and directed me to both rheumatoid arthritis and OB doctors.” Another woman described that her providers’ opinions appeared to be influenced by how familiar they were with DMARDs: “Even two different high‐risk specialists had different opinions. Obstetrician overstated prednisone risks and didn’t understand 3rd trimester risks with [rituximab]. Rheumatologist was the most evidence‐based. PCP thought I should quit taking (everything).”
Women independently sought information about medication safety during pregnancy and lactation (Figure 3). Most frequently, women used online health searches (59%); whereas some used reliable websites, such as WebMD and the Arthritis Foundation, nearly half perused less reliable sites, including blogs and social media sources.
Figure 3.

Online and other resources for women seeking information on medication safety during pregnancy and lactation.
DISCUSSION
In this survey of reproductive‐aged women with inflammatory arthritis, women were hesitant to use any medications during pregnancy or while breastfeeding. Women pregnant after 2012, as compared to earlier years, were more likely to receive conflicting medical advice about DMARD safety but were more likely to use TNFis while pregnant or lactating. Fifteen percent of pregnancies in this sample were exposed to methotrexate, a medication with fetotoxic potential. Our qualitative analysis highlighted the tensions women felt between prioritizing the health and safety of their children and their own physical functioning.
Most women in this sample discontinued safe DMARDs in preparation for or during pregnancy, a higher percentage of medication discontinuation than has been reported in previous studies that have primarily relied on pharmacy data or chart review (31%‐62%) (1, 2, 3). Because most women in this sample could not correctly identify pregnancy‐compatible medications, knowledge gaps about medication safety may have prompted some women to discontinue their medications. These knowledge gaps may have been potentiated by the use of unverified online resources by a majority of participants, which has been described in other work (27).
Health care providers may have also contributed to misinformation: nearly one‐quarter of women expressed that their providers gave them conflicting advice about medication safety. Our exploratory analysis suggested that after 2012, women increasingly received conflicting advice from health care providers regarding medication safety. Most physicians may have counseled women to discontinue all medications except for prednisone or NSAIDs before 2012, a year that marked the publication of multiple review articles on medication safety. It is possible that after 2012, some physicians may have been familiar with emerging safety data for certain DMARDs in pregnancy, whereas others relied on their prior perceptions of risk, thereby leading to inconsistencies in medical advice. Our findings suggest that conflicting medical advice between providers augmented patients’ concerns about medication safety; other studies have suggested that such conflicts may lead patients to discontinue safe medications unnecessarily (28, 29).
The impact of conflicting medical advice on patients’ trust in their providers and comfort level with their medications is particularly relevant because of the recent publication of the inaugural ACR reproductive health guideline for patients with rheumatic diseases (17). Guideline adoption is often slow and inconsistent across health care providers, especially across disciplines (30, 31). Thus, in the context of the publication of the reproductive health guideline, some patients may be at even greater risk of receiving conflicting medical advice about medication safety. In addition to reviewing the reproductive health guideline, providers can learn more about medication safety in pregnancy and lactation by using other evidence‐based resources, such as the Healthy Outcomes in Pregnancy with SLE Through Education of Providers (HOP‐STEP) (32) and the Organization of Teratology Information Specialists (33). Rheumatologists must also be proactive in reaching consensus about medication safety with providers with whom they comanage patients so that patients receive coherent, consistent, and evidence‐based information from their medical team.
Our qualitative analysis highlighted women’s fears about how DMARD exposure might affect their children. Many participants experienced disease flares in the postpartum period—a well‐documented phenomenon in inflammatory arthritis (5, 34, 35)—and their responses often reflected a willingness to suffer physically rather than to expose their infants to DMARDs, no matter how safe. Although not studied herein, social norms and expectations around motherhood that prioritize the child’s health and well‐being may factor into women’s decisions to discontinue medications during pregnancy and lactation (36, 37, 38). Women at increased risk of pregnancy complications related to their arthritis may benefit from the knowledge that to discontinue medications is not necessarily a neutral choice; because uncontrolled maternal disease activity may have implications on fetal health, their self‐sacrifice may not necessarily lead to optimal pregnancy and fetal outcomes. Rheumatologists may also reassure patients that many arthritis medications are safe during pregnancy (14, 15, 16, 17, 39, 40) and that drug transfer between a breastfeeding mother and baby is generally less than drug transfer during pregnancy (41, 42).
Fifteen percent of pregnancies in this sample were exposed to methotrexate, a fetotoxic DMARD (14, 15). We expect that most women who conceived while using methotrexate became pregnant unintentionally and took the medication because they did not realize they were pregnant or did not realize that methotrexate was fetotoxic. This finding highlights a persistent and concerning gap in anticipatory family planning care for women with rheumatic diseases (43, 44).
This study has several strengths. Because we worked closely with a patient group to develop the survey, we believe that our findings may be of particular interest to reproductive‐aged women with inflammatory arthritis. Although we were unable to independently verify women’s diagnoses of inflammatory arthritis, 95% had used a DMARD or prednisone, which suggests that our sample was valid.
Limitations of our study included that we were unable to assess how patients’ medication decision‐making might affect objective clinical or fetal outcomes, an important factor in contextualizing the extent to which high rates of pregnancy morbidity among women with inflammatory arthritis are related to potentially reversible factors. Furthermore, our study sample primarily consisted of White women of high socioeconomic status who had Internet access; more information is needed to capture how a more representative population of women with rheumatic diseases assesses medication risk in the context of pregnancy. In addition, only a small number of ArthritisPower and CreakyJoints members who were invited to participate appeared to enroll in the study. However, our recruitment methods may have led to an artificially low response rate. Although our inclusion criteria limited participation to women between the ages of 18 and 50, email solicitations were sent to ArthritisPower and CreakyJoints members without restriction. Because CreakyJoints does not collect the ages of its members, we are unsure how many female members who received the email were ineligible for our study. Other reasons for a potentially low response rate might include the length of the survey and the fact that we did not compensate respondents for completing the survey. It is therefore possible that women who completed our survey were particularly motivated to participate on the basis of prior adverse experiences or concerns related to reproductive health, leading to a form of response bias. However, congruent with qualitative analysis principles, which prioritize in‐depth exploration of a topic rather than generalizability, our mixed‐method analysis highlights important perspectives from women who were particularly concerned about reproductive health (45). We believe that our analysis provides nuance and depth about these women’s experiences and may help rheumatologists and other health care providers to better anticipate and address the information needs and priorities of this group.
In conclusion, this study found that most women with inflammatory arthritis discontinued medications around pregnancy and lactation because of concerns about medication risks. The introduction of new data about medication safety appeared to coincide with conflicting advice of health care providers and appeared to undermine some women’s trust in medication safety and in their providers. Our findings underscore the importance of care coordination between health care providers so that messaging about medication safety is consistent and evidence based. High‐quality information is needed for patients and providers alike to support informed, up‐to‐date medication decision‐making that preserves women’s physical functioning and well‐being through all phases of their reproductive lives.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Birru Talabi, Eudy, and Clowse had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Eudy, Nowell, Curtis, Crow‐Hercher, White, Ginsberg, Clowse.
Acquisition of data
Eudy, Jayasundara, Haroun, Clowse.
Analysis and interpretation of data
Birru Talabi, Eudy, Jayasundara, Haroun, Nowell, Curtis, Crow‐Hercher, White, Ginsberg, Clowse.
Acknowledgments
We would like to thank the CreakyJoints and ArthritisPower members for their generous and thoughtful contributions to all aspects of this study.
Supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grant K23‐AR‐075057.
Dr. Nowell owns stock in AbbVie, Alexion, Allergan, Biogen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Eli Lilly, Merck, Novartis, Pfizer, and UCB. Dr. Curtis has received research grants (paid to The University of Alabama at Birmingham) from AbbVie, Amgen, Bristol Myers Squibb, Corrona, Eli Lilly, Janssen, Myriad, Pfizer, Radius, Regeneron, Roche, and UCB (more than $10,000 each). He has received consulting fees from Amgen, Corrona, Eli Lilly, Myriad, and Pfizer (more than $10,000 each) and from AbbVie, Bristol Myers Squibb, Janssen, Radius, Regeneron, Roche, and UCB (less than $10,000 each). Dr. White is on the speaker’s board for AbbVie, Inc. and receives compensation for presentations given (approximately $5,000 per year). Dr. Clowse has received consulting fees from UCB (more than $10,000) and research grants (paid to Duke University) (more than $10,000). No other disclosures relevant to this article were reported.
REFERENCES
- 1. Desai RJ, Huybrechts KF, Bateman BT, Hernandez‐Diaz S, Mogun H, Gopalakrishnan C, et al. Brief report: patterns and secular trends in use of immunomodulatory agents during pregnancy in women with rheumatic conditions. Arthritis Rheumatol 2016;68:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsao NW, Lynd LD, Sadatsafavi M, Hanley G, de Vera MA. Patterns of biologics utilization and discontinuation before and during pregnancy in women with autoimmune diseases: a population‐based cohort study. Arthritis Care Res (Hoboken) 2018;70:979–86. [DOI] [PubMed] [Google Scholar]
- 3. Kuriya B, Hernandez‐Diaz S, Liu J, Bermas BL, Daniel G, Solomon DH. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:721–8. [DOI] [PubMed] [Google Scholar]
- 4. de Man YA, Hazes JM, van der Heide H, Willemsen SP, de Groot CJ, Steegers EA, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum 2009;60:3196–206. [DOI] [PubMed] [Google Scholar]
- 5. Barrett JH, Brennan P, Fiddler M, Silman AJ. Does rheumatoid arthritis remit during pregnancy and relapse postpartum? Results from a nationwide study in the United Kingdom performed prospectively from late pregnancy. Arthritis Rheum 1999;42:1219–27. [DOI] [PubMed] [Google Scholar]
- 6. Jethwa H, Lam S, Smith C, Giles I. Does rheumatoid arthritis really improve during pregnancy? A systematic review and metaanalysis. J Rheumatol 2019;46:245–50. [DOI] [PubMed] [Google Scholar]
- 7. Chen JS, Ford JB, Roberts CL, Simpson JM, March LM. Pregnancy outcomes in women with juvenile idiopathic arthritis: a population‐based study. Rheumatology (Oxford) 2013;52:1119–25. [DOI] [PubMed] [Google Scholar]
- 8. Smith CJ, Forger F, Bandoli G, Chambers CD. Factors associated with preterm delivery among women with rheumatoid arthritis and juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2019;71:1019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bharti B, Lee SJ, Lindsay SP, Wingard DL, Jones KL, Lemus H, et al. Disease severity and pregnancy outcomes in women with rheumatoid arthritis: results from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project. J Rheumatol 2015;42:1376–82. [DOI] [PubMed] [Google Scholar]
- 10. Zbinden A, van den Brandt S, Ostensen M, Villiger PM, Forger F. Risk for adverse pregnancy outcome in axial spondyloarthritis and rheumatoid arthritis: disease activity matters. Rheumatology (Oxford) 2018;57:1235–42. [DOI] [PubMed] [Google Scholar]
- 11. Brouwer J, Fleurbaaij R, Hazes JM, Dolhain RJ, Laven JS. Subfertility in women with rheumatoid arthritis and the outcome of fertility assessments. Arthritis Care Res (Hoboken) 2017;69:1142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brouwer J, Hazes JM, Laven JS, Dolhain RJ. Fertility in women with rheumatoid arthritis: influence of disease activity and medication. Ann Rheum Dis 2015;74:1836–41. [DOI] [PubMed] [Google Scholar]
- 13. De Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum 2008;59:1241–8. [DOI] [PubMed] [Google Scholar]
- 14. Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding. Part I. Standard and biologic disease modifying anti‐rheumatic drugs and corticosteroids. Rheumatology (Oxford) 2016;55:1693–7. [DOI] [PubMed] [Google Scholar]
- 15. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer‐Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. [DOI] [PubMed] [Google Scholar]
- 16. Kavanaugh A, Cush JJ, Ahmed MS, Bermas BL, Chakravarty E, Chambers C, et al. Proceedings from the American College of Rheumatology Reproductive Health Summit: the management of fertility, pregnancy, and lactation in women with autoimmune and systemic inflammatory diseases. Arthritis Care Res (Hoboken) 2015;67:313–25. [DOI] [PubMed] [Google Scholar]
- 17. Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse ME, Lockshin MD, et al. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020;72:529–56. [DOI] [PubMed] [Google Scholar]
- 18. Birru Talabi M, Eudy AM, Jayasundara M, Haroun T, Nowell WB, Curtis JR, et al. Pregnancy, periods, and “the pill”: exploring the reproductive experiences of women with inflammatory arthritis. ACR Open Rheumatol 2019;1:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raja H, Matteson EL, Michet CJ, Smith JR, Pulido JS. Safety of tumor necrosis factor inhibitors during pregnancy and breastfeeding. Transl Vis Sci Technol 2012;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen OH, Jess T. IBD: can TNF inhibitors be administered during the third trimester? [review]. Nat Rev Gastroenterol Hepatol 2013;10:130–1. [DOI] [PubMed] [Google Scholar]
- 21. Chambers CD, Johnson DL. Emerging data on the use of anti‐tumor necrosis factor‐α medications in pregnancy. Birth Defects Res A Clin Mol Teratol 2012;94:607–11. [DOI] [PubMed] [Google Scholar]
- 22. Murdaca G, Colombo BM, Cagnati P, Gulli R, Spano F, Puppo F. Update upon efficacy and safety of TNF‐α inhibitors. Expert Opin Drug Saf 2012;11:1–5. [DOI] [PubMed] [Google Scholar]
- 23. Abarientos C, Sperber K, Shapiro DL, Aronow WS, Chao CP, Ash JY. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin Drug Saf 2011;10:705–14. [DOI] [PubMed] [Google Scholar]
- 24. Diav‐Citrin O, Blyakhman S, Shechtman S, Ornoy A. Pregnancy outcome following in utero exposure to hydroxychloroquine: a prospective comparative observational study. Reprod Toxicol 2013;39:58–62. [DOI] [PubMed] [Google Scholar]
- 25. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs 2008;62:107–15. [DOI] [PubMed] [Google Scholar]
- 26. Pope C, Ziebland S, Mays N. Qualitative research in health care: analysing qualitative data. BMJ 2000;320(7227):114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chew C, Rebic N, Baldwin C, Amiri N, Proulx L, de Vera MA. "r/Thritis", pregnancy, and parenting: a qualitative descriptive study of reddit forums to explore information needs and concerns of women with rheumatoid arthritis. ACR Open Rheumatol 2019;1:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hameen‐Anttila K, Nordeng H, Kokki E, Jyrkka J, Lupattelli A, Vainio K, et al. Multiple information sources and consequences of conflicting information about medicine use during pregnancy: a multinational Internet‐based survey. J Med Internet Res 2014;16:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carpenter DM, Elstad EA, Blalock SJ, DeVellis RF. Conflicting medication information: prevalence, sources, and relationship to medication adherence. J Health Commun 2014;19:67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lomas J. Words without action? The production, dissemination, and impact of consensus recommendations. Annu Rev Public Health 1991;12:41–65. [DOI] [PubMed] [Google Scholar]
- 31. Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta‐review. BMC Med Inform Decis Mak 2008;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Njagu R, Criscione‐Schreiber LG, Eudy A, Snyderman A, Clowse MEB. Impact of a Multifaceted Educational Program to Improve Provider Skills for Lupus Pregnancy Planning and Management: a mixed‐methods approach. ACR Open Rheumatol 2020;2:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oganization of Teratology Information Specialists . Mother to Baby. URL: https://mothertobaby.org
- 34. Barrett JH, Brennan P, Fiddler M, Silman A. Breast‐feeding and postpartum relapse in women with rheumatoid and inflammatory arthritis. Arthritis Rheum 2000;43:1010–5. [DOI] [PubMed] [Google Scholar]
- 35. Genest G, Spitzer KA, Laskin CA. Maternal and fetal outcomes in a cohort of patients exposed to tumor necrosis factor inhibitors throughout pregnancy. J Rheumatol 2018;45:1109–15. [DOI] [PubMed] [Google Scholar]
- 36. McDonald K, Amir LH, Davey MA. Maternal bodies and medicines: a commentary on risk and decision‐making of pregnant and breastfeeding women and health professionals. BMC Public Health 2011;11 Suppl 5:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rizzo KM, Schiffrin HH, Liss M. Insight into the parenthood paradox: mental health outcomes of intensive mothering. J Child Fam Stud 2013;22:614–20. [Google Scholar]
- 38. Hays S. The cultural contradictions of motherhood. New Haven (CT): Yale University Press; 1998. [Google Scholar]
- 39. Birru Talabi M, Clowse ME, Schwarz EB, Callegari LS, Moreland L, Borrero S. Family planning counseling for women with rheumatic diseases. Arthritis Care Res (Hoboken) 2018;70:169–74. [DOI] [PubMed] [Google Scholar]
- 40. Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding. Part II. analgesics and other drugs used in rheumatology practice. Rheumatology (Oxford) 2016;55:1698–702. [DOI] [PubMed] [Google Scholar]
- 41. Janssen NM, Genta MS. The effects of immunosuppressive and anti‐inflammatory medications on fertility, pregnancy, and lactation. Arch Intern Med 2000;160:610–9. [DOI] [PubMed] [Google Scholar]
- 42. Birru Talabi M, Clowse ME. Antirheumatic medications in pregnancy and breastfeeding. Curr Opin Rheumatol 2020;32:238–46. [DOI] [PubMed] [Google Scholar]
- 43. Schwarz EB, Manzi S. Risk of unintended pregnancy among women with systemic lupus erythematosus. Arthritis Rheum 2008;59:863–6. [DOI] [PubMed] [Google Scholar]
- 44. El Miedany Y, Palmer D. Rheumatology‐led pregnancy clinic: enhancing the care of women with rheumatic diseases during pregnancy. Clin Rheumatol 2020;39:3593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carminati L. Generalizability in qualitative research: a tale of two traditions. Qual Health Res 2018;28:2094–101. [DOI] [PubMed] [Google Scholar]


