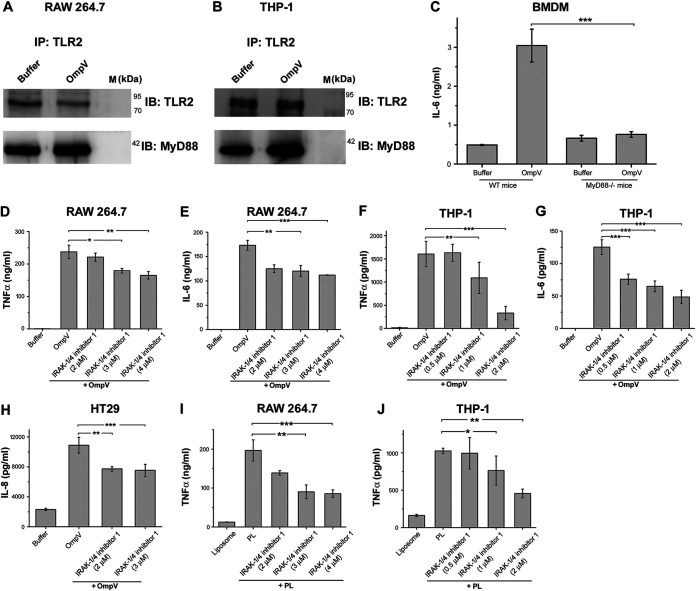
FIG 6.
MyD88 and IRAK are involved in OmpV-mediated signaling in macrophages, monocytes, and IECs. (A, B) Increased association of MyD88 with TLR2 in OmpV-activated macrophages and monocytes. Cell lysates of OmpV-treated RAW 264.7 macrophages (A) and THP-1 monocytes (B) were immunoprecipitated with anti-TLR2 antibody and checked for the presence of MyD88. Buffer-treated cells were used as controls. IP indicates the antibody used for immunoprecipitation, whereas IB indicates the antibody used for immunoblotting. Western blots are representatives of three independent experiments. (C) A significant decrease in proinflammatory cytokine production was observed in macrophages under MyD88-deficient conditions. BMDMs from wild-type and MyD88−/− mice were treated with PmB followed by OmpV. Following incubation, supernatants were collected and analyzed for IL-6 production. (D to G) A significant decrease in proinflammatory cytokine production was observed with inhibition of IRAK-1/4 in macrophages and monocytes. (H) A significant decrease in IL-8 was observed upon inhibition of IRAK-1/4 in IECs. (I, J) IRAK is involved in OmpV-proteoliposome (PL)-mediated signaling. (D to J) RAW 264.7 macrophages, THP-1 monocytes, or HT29 cells were pretreated with IRAK-1/4 inhibitor followed by treatment with PmB and OmpV or OmpV-proteoliposome (PL). Following incubations, supernatants were collected and analyzed for cytokine production by ELISA. Bar graphs are expressed as mean ± SEM from three independent experiments; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05 versus the OmpV-activated BMDMs from wild-type mice (C), versus only OmpV-treated cells (D to H), or versus only OmpV-proteoliposome (PL)-treated cells (I, J).