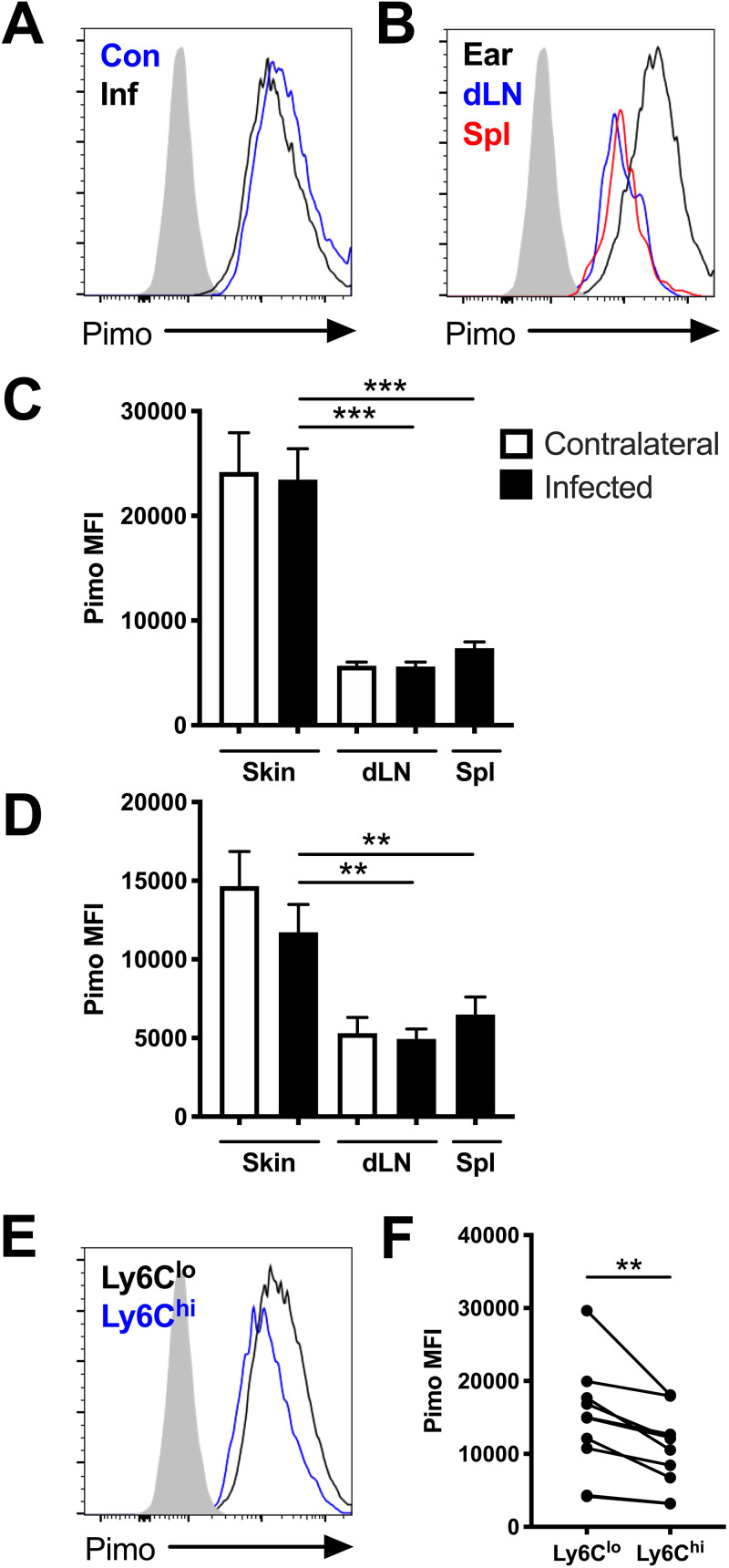
FIG 1.
Macrophages experience hypoxic conditions within the site of infection following L. major inoculation in vivo. C57BL/6 mice were infected with L. major parasites intradermally in the ear. At 1 and 5 weeks p.i., infected and contralateral ears, infected and contralateral dLNs, and spleens were stained with pimonidazole and analyzed by flow cytometry. (A) Representative flow cytometry histograms showing pimonidazole (pimo) median fluorescence intensity (MFI) after gating on total, live, singlet, and CD45+ CD11b+ Ly6G− for F4/80+ or CD64+ macrophages from infected (Inf) and contralateral (Con) ears from the same mouse. (B) Representative flow cytometry histograms showing pimonidazole MFI after gating on F4/80+ macrophages from the infected ear or corresponding dLN and spleen of the same mouse. (C) Quantification of pimonidazole MFI in macrophages from the skin, dLN, and spleen at 1 week p.i. (D) Quantification of pimonidazole MFI in macrophages from the skin, dLN, and spleen at 5 weeks p.i. (E) Representative flow cytometry histograms showing pimonidazole MFI after gating on macrophages from infected skin that were Ly6Chi or Ly6Clo at 5 week p.i. (F) Quantification of pimonidazole MFI for Ly6Chi or Ly6Clo macrophages from infected ears from panel E. Fluorescence minus one (FMO) controls for pimonidazole staining are seen in gray (A, B, E). Data shown were pooled from 2 experiments with 5 mice per group per time point (n = 10 mice per condition). Data are presented as the mean plus standard error of the mean (SEM). **, P < 0.01; ***, P < 0.001, paired t test comparing macrophages from infected skin to dLNs or spleens or comparing dermal macrophages with variable Ly6C expression.