Figure 1.
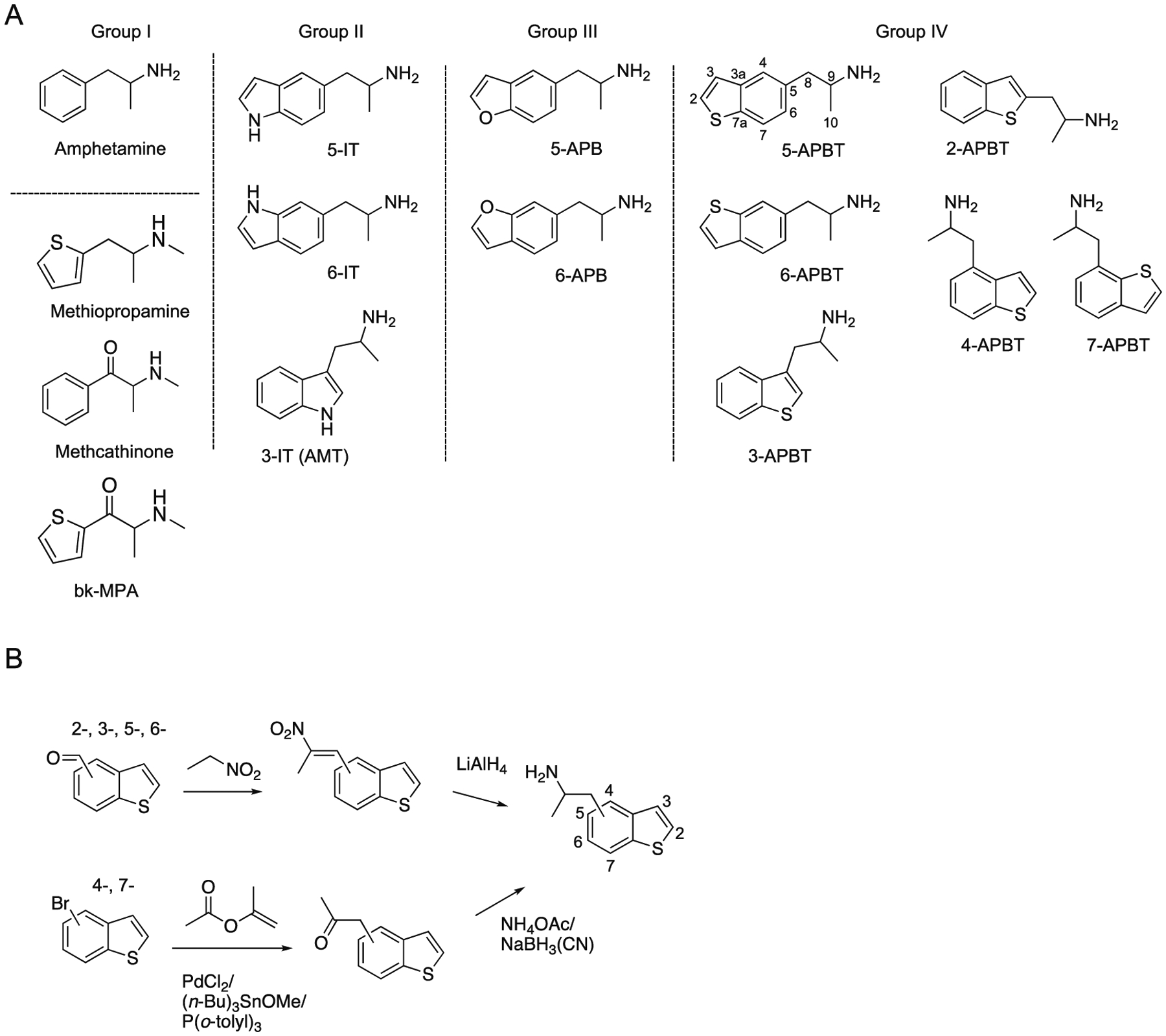
A. Chemical structures of the classic psychostimulant amphetamine with methiopropamine reflecting the bioisosteric counterpart of methamphetamine. The corresponding sulfur analog of methcathinone gives rise to bk-methiopropamine (group I). Examples of three 5-(2-aminopropyl)indoles (AMT, 5-IT and 6-IT, group II) and two (2-aminopropyl)benzofuran isomers 5-APB and 6-APB (group III) that also became prominent new psychoactive substances in recent years. Group IV. Six novel benzo[b]thiophene analogs being subject of the present investigation. B. Generalized synthesis scheme used for the preparation of all six APBT isomers.
