Fig. 1 |. Artificial intrinsically disordered polypeptides inspired from native IDPs exhibit reversible UCST phase behaviour.
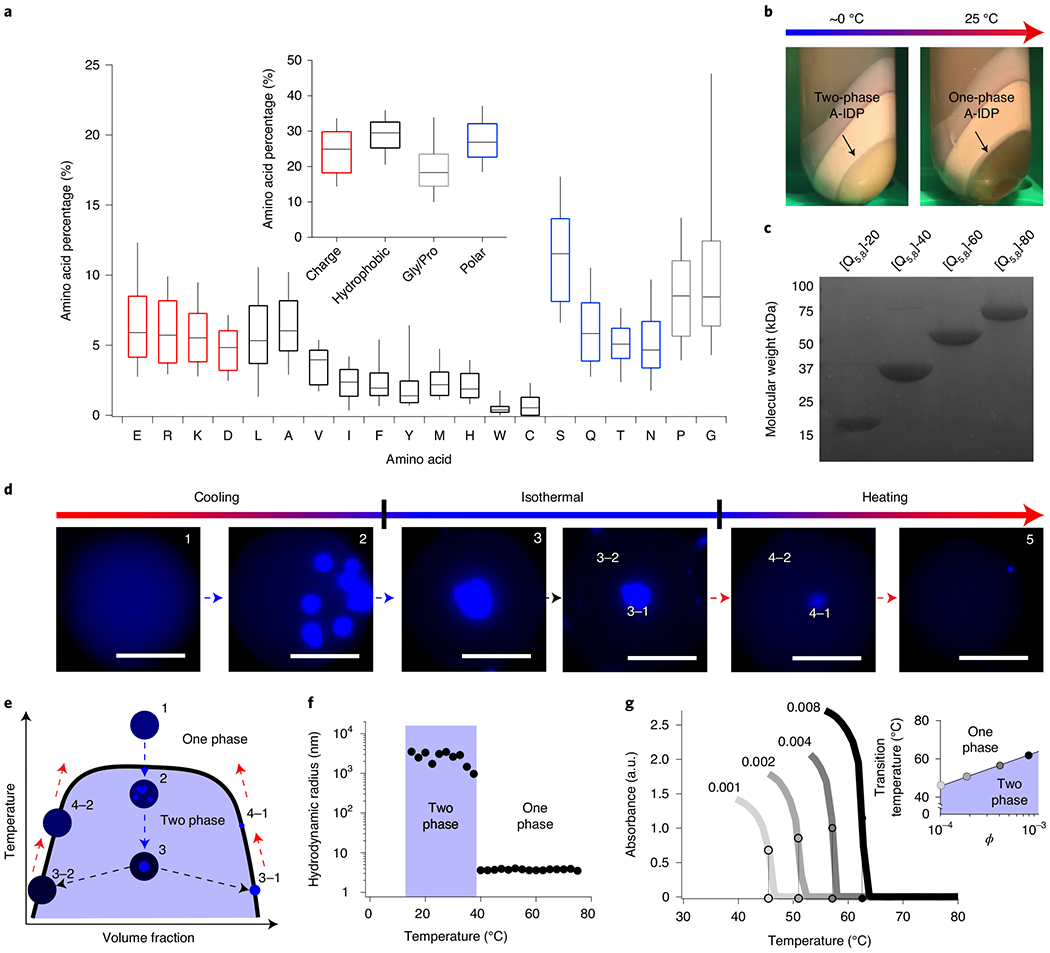
a, Proteomic analysis of native IDPs that form biomolecular condensates reveal that they have an abundance of G/P, charged and uncharged polar residues, yet exhibit a balance of overall charge. b, An example of a dense, exclusionary phase formed by an UCST-exhibiting A-IDP even in the complex medium of bacterial cell lysate. The coacervate shows almost complete separation from all other cellular proteins and debris (non-labelled bands) that are present in the cell lysate after centrifugation, facilitating purification of the A-IDP from the insoluble cell lysate fraction without affinity tags. Even in this concentrated phase, one can still identify the A-IDP with optical turbidity as it transitions between a two- and one-phase solution on heating. c, An example SDS-PAGE gel of a set of A-IDPs ([Q5,8]-20 to [Q5,8]-80) with conserved sequence but increasing molecular weight, showing the high purity of the A-IDPs that is obtained by exploiting their UCST phase behaviour for chromatography-free purification. d, Visualization of UCST phase separation of [Q5,8]-20 in water-in-oil droplets by fluorescence microscopy. On cooling, phase separation in a droplet is initiated at multiple sites; the puncta that grow from each site slowly coalesce with one another into a single dense phase. The equilibrium with the surrounding dilute phase is re-established on reheating, leading to a higher-concentration dilute phase and smaller volume occupied by the dense phase. ϕ = 0.0018 (100 μM); scale bar, 50 μm. e, A schematic UCST phase diagram for a cooling-heating cycle of a UCST polypeptide in a water-in-oil droplet. f, Dynamic light scattering data of [Q5,8]-20, demonstrating the change in the hydrodynamic radius following cooling. On reaching the cloud point, [Q5,8]-20 transitions from soluble unimeric polypeptides with a radius of hydration of 5–6 nm to micron-sized aggregates. Data are collected at ϕ = 0.0043 (245 μM) in 150 mM PBS at pH 7.4. g, Upper critical solution temperature cloud points are affected by ϕ of the polypeptide in solution. The inset shows that this behaviour follows a natural-logarithm dependence in the dilute regime (R2 = 0.98).
