Fig. 6 |. Engineered intracellular droplets with programmable function.
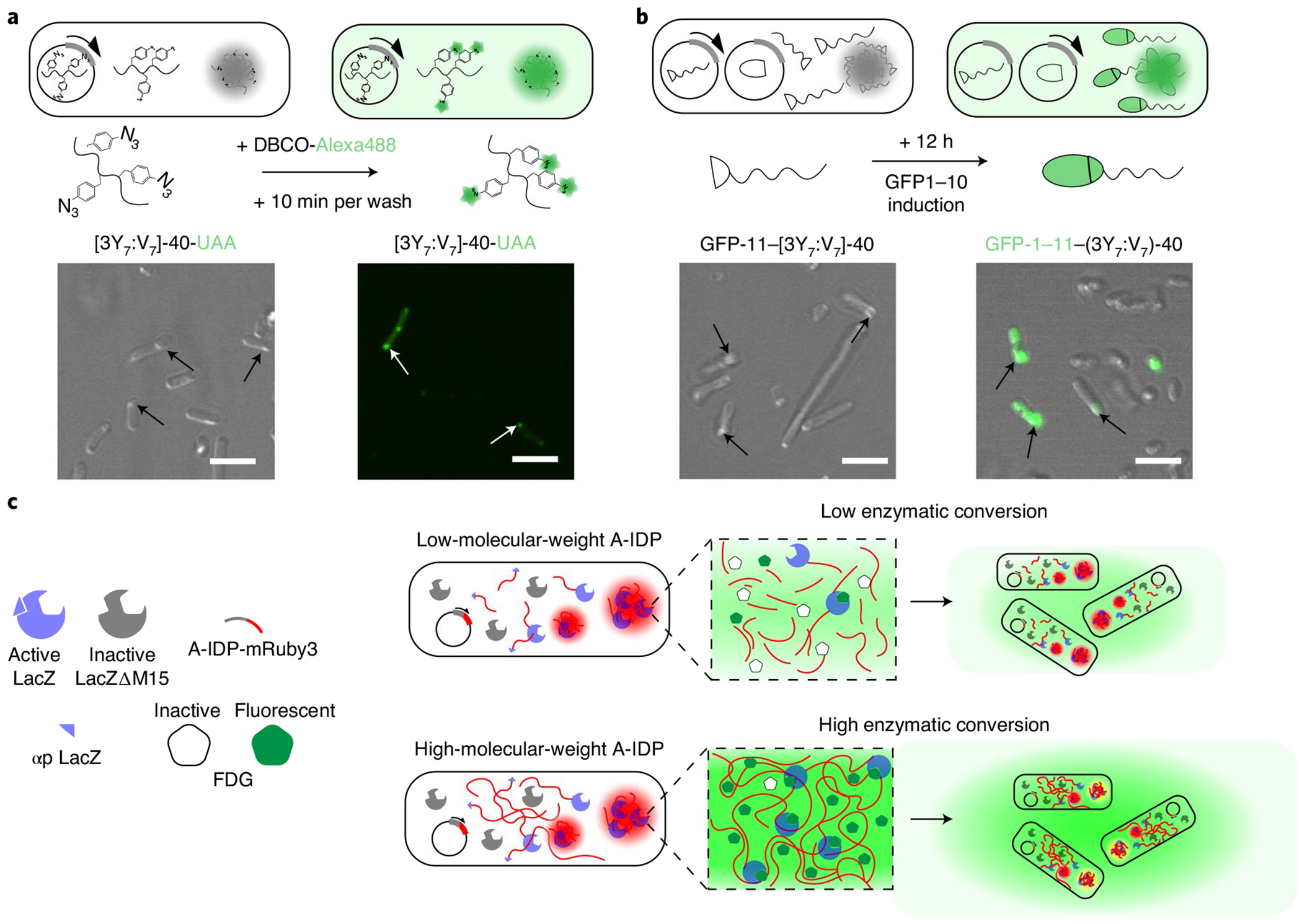
a, Site-specific labelling of droplets with a small molecule fluorescent dye. E. coli cells that contain condensates that are formed by a [3Y7:V7]-40 variant with azidophenylalanine (AzF) residues present a biorthogonal azide that can be labelled in situ with DBCO-Alexa488. DBCO-Alexa488 mixture can diffuse into cells and into the A-IDP condensates within the cell, labelling the azide groups within 10 min of incubation with live E. coli. b, Reconstitution of function GFP in condensates by recruitment of a partner from the cytoplasm using a split GFP system. A GFP-11–[3Y7:V7]-40 fusion protein is able to recruit GFP-1–10 from the surrounding cytoplasm into intracellular droplets. On formation of intracellular condensates after 24 h of IPTG induction of GFP-11–[3Y7:V7]-40 (left panel), induction of GFP-1–10 by arabinose induction enables recruitment of GFP-1–10 into the condensates and reconstitution of functional GFP-1–11 within existing intracellular condensates within 12 h of GFP-1–10 induction (right panel). c, A schematic of the enzyme-condensate experiment. The αp of LacZ is fused with a fluorescent reporter protein (mRuby3) and expressed from an IPTG-inducible gene from a plasmid in the E. coli strain KRX, which has a deletion mutant of the LacZ gene that produces a truncated, catalytically inactive enzyme that lacks the αp. Complementation of LacΔM15 by a αp–A-IDP–mRuby3 fusion creates an active enzyme that converts FDG into fluorescein that is then rapidly exported from the intracellular space into the surrounding medium.
