Abstract
At the end of 2019, a new disease with pandemic potential appeared in China. It was a novel coronavirus called coronavirus disease 2019 (COVID-19). Later, in the first quarter of 2020, the World Health Organization declared the outbreak of this disease a pandemic. Elderly people, people with comorbidities, and health care professionals are more vulnerable to COVID-19. Obesity has been growing exponentially worldwide, affecting several age groups. It is a morbidity that is associated with genetic, epigenetic, environment factors and/or interaction between them. Obesity is associated with the development of several diseases including diabetes mellitus, mainly type 2. Diabetes affects a significant portion of the global population. Obesity and diabetes are among the main risk factors for the development of severe symptoms of COVID-19, and individuals with these conditions constitute a risk group. Based on a literature review on obesity in people with diabetes in the framework of the COVID-19 pandemic, this study presents updated important considerations and care to be taken with this population.
Keywords: Obesity, Diabetes, COVID-19, Population, World, Disease
Core Tip: This article addresses obesity in people with diabetes in coronavirus disease 2019 (COVID-19) times, addressing the main points of the diseases and the interaction between them. Based on a literature review on obesity in people with diabetes in the framework of the COVID-19 pandemic, this study presents updated important considerations and care to be taken with this population.
INTRODUCTION
Obesity affects people of different age groups, with a significant increase in its prevalence in the last three decades, with worldwide prevalence rates of 27.5% in adults and 47.1% in children[1]. Obesity is associated with the occurrence of several diseases, including diabetes mellitus (DM)[2]. Obesity is involved not only in the etiopathogenesis of the most common type of diabetes, type 2 DM but also in its complications. There is also a growing scientific evidence on the role of obesity in type 1 diabetes[3].
Diabetes affects more than 422 million adults worldwide, which is predicted to increase to 640 million by 2040[4,5]. It is one of the major causes of mortality and reduced life expectancy worldwide[6]. Among the types of diabetes, type 1 is an autoimmune disease mediated by T cells, marked by an insulin defect resulting from the destruction of the pancreatic β cells[7], with an estimated prevalence of 9.5% worldwide[8]. Type 2 diabetes is a condition that involves resistance to the effects of insulin or an inefficient production of insulin to maintain a normal blood glucose level[8]. Corresponding to more than 90% of diabetes cases, type 2 diabetes has an estimated prevalence of 9.3% and is growing exponentially, in parallel with the increase in obesity[9].
In March 2020, the World Health Organization (WHO) declared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a pandemic after the virus had infected more than 381000 people in 195 countries[10]. The course of the disease is varied, depending on individual immunological factors, age, and existing comorbidities. Individuals with obesity and diabetes need greater care in times of coronavirus disease 2019 (COVID-19), since they are part of the risk groups[11] and the subsequent development of the infection has been highly associated with morbidity and mortality in these individuals[12-14].
Based on a literature review on obesity in people with diabetes in times of the COVID-19 pandemic, this study presents updated important considerations and care to be taken with this population.
OBESITY
Obesity is one of the most serious public health problems of the 21st century[15]. It is a complex multifactorial disease that affects individuals of all age groups in low- and middle-income countries in urban areas[15,16]. Many studies have shown that the main cause of obesity is calorie consumption greater than energy expenditure, resulting in high energy storage in the form of fat, especially in the abdominal region, associated with disorders such as type 2 DM[17], metabolic syndrome[18], obstructive sleep apnea[19], and increased risk of cardiovascular disease[18], conditions that decrease life expectancy.
The worldwide prevalence of overweight and obesity has doubled since 1980, so that almost one-third of the world population is classified as overweight or obese. The significant increase in the incidence of obesity in recent years makes this disease an epidemic[20,21]. According to data from the Global Burden of Disease[20,21], obesity rates increased at all ages and in both genders, regardless of geographic location, ethnicity or socioeconomic status, although the prevalence of obesity is generally higher in the elderly and in women. This trend was similar in all regions and countries, although the rates of absolute prevalence of overweight and obesity varied widely. In some developed countries, prevalence rates appear to have stabilized in recent years. However, in the United States, according to 2019[5] data from the Centers for Disease Control and Prevention, six states had a 35% or more obesity prevalence among non-Hispanic white adults; 15 states had a prevalence of 35% or more among Hispanic adults and in 34 states and the District of Columbia, the prevalence of obesity was 35% or more among non-Hispanic black adults[22].
Recent data from the WHO[23] showed that in the past four decades, the number of children and adolescents with obesity has increased considerably worldwide. While just under 4% of children and adolescents aged 5 to 19 years were obese in 1975, more than 124 million were classified as obese in 2016 that is, the prevalence that was 4% in 1975 increased to 18% in about 40 years. This is critical, since the probability of an obese child remaining obese in adulthood ranges from 20% to 50%. For an adolescent with obesity, this probability increases even more[24,25].
In view of the complexity of obesity, its pathophysiological aspects and also the importance of this chronic disease as a public health problem, there has been a growing number of studies aimed at understanding the pathophysiology of this disease and associated events. In this connection, it is important to note that the white adipose tissue (WAT) is the main secretory site of leptin, an adipokine, which upon reaching the central nervous system (CNS), acts on the hypothalamic receptors and reduces appetite. However, high levels of leptin in individuals with obesity, cause a process of central resistance of this hormone, impairing the signs of satiety[26]. In addition, another hormone present in the metabolic homeostasis is insulin, which is stimulated by increased glucose in the bloodstream. However, changes in eating behavior can be associated with an imbalance of this hormone. For example, the excess of free fatty acids directly affects cellular metabolism, triggering the activation of inflammatory pathways, and therefore, the development of insulin resistance[27].
In contrast, the high consumption of nutrients, exceeding the body's energy needs, can generate adipocytes hypertrophy and hyperplasia[28,29], and consequently, the compression of blood vessels, hindering adequate oxygen supply, generating hypoxia[30,31]. Hypoxia is related to the recruitment of macrophages for this tissue[32-36]. The infiltrated macrophages form crown-like structures surrounding the adipocytes, which leads to excessive adipokine production, which includes pro-inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, C-reactive protein (CRP), and adipokines[37-44].
Thus, obesity is characterized by a low-grade chronic inflammation, detected by the elevation of pro-inflammatory mediators and by the presence of macrophages infiltrated in WAT[45,46]. This inflammation in WAT turns into systemic inflammation and contributes to the onset and progression of associated metabolic diseases[47-49]. In addition, studies conducted in the last decade have shown that obesity and its comorbidities are not limited to peripheral tissues, but also involve the CNS[50-53].
DM
DM is defined as a disorder in the glucose metabolism, in which the serum glucose concentration is abnormally high, as the body does not release or use glucose properly[54]. The two most common forms of diabetes are type 1 and type 2. Type 1 diabetes results from the autoimmune destruction of the pancreatic beta cells, which produce insulin, and type 2 diabetes is caused mainly by a combination of insulin resistance and relative insulin deficiency[55].
DM, as well as obesity, is also considered one of the major public health problems of the 21st century. In 2019, there were 463 million people with diabetes in the world, expected to increase to 700 million by 2045[56]. This increase in the prevalence of diabetes is due to the epidemiological and nutritional transition, population aging, physical inactivity, overweight and obesity, and further to the greater survival of individuals with diabetes[54].
The countries with the highest prevalence of DM in adults aged 20 to 79 years in the world are China, India and the United States. The region of Central and South America ranks fifth, with 32 million people with the disease. Brazil appears in the sixth place, with 16.8 million people with DM in 2019 and an estimate to reach 26 million by 2045[56].
The prevalence of DM in this country varies according to the survey carried out, and according to the region and age group assessed: In the Social Dimensions of Inequalities Survey, carried out in 2008 with the representation of macro-regions, a national prevalence of 7.5% self-reported DM was observed[57]. The National Health Survey (NHS), carried out in 2013, indicated a 6.2% prevalence of self-reported diabetes, that is, the previous diagnosis of the disease was reported by more than 9 million Brazilians aged 18 or over[58]. In a subsample of the NHS with collection of biochemical tests, carried out in 2014/2015, the prevalence of DM based on glycated hemoglobin data was 6.6% and, even considering the use of medication, 8.4%[59]. Data indicated a progressive increase in this morbidity reported in the adult population, from 1980 to 2015, in both men and women[60]. In different analyses, diabetes is associated with older age, less education, and overweight/obesity[58,59].
Diabetes is a chronic condition that requires constant care, including weight control through healthy habits, such as low carbohydrate diet, regular physical activity and, in some cases, help with hypoglycemic medication. Adequate glycemic control can delay or prevent the occurrence of microvascular and macrovascular complications of the disease, especially in patients diagnosed at an earlier age, with repercussions on the patient's quality of life and general health, such as retinopathy, nephropathy, neuropathy, coronary disease, cerebrovascular disease and peripheral arterial disease[54].
One of the problems associated with DM is the increased susceptibility to infections, due to several factors, directly or indirectly related to chronic hyperglycemia such as reduced vascular response to inflammatory mediators such as histamine and bradykinin, decreased activity of neutrophil polymorphonuclear cells, alteration in adherence, chemotaxis and leukocyte opsonization, peripheral and autonomic neuropathy, decreased protein binding with consequent edema, inefficient and delayed cellular immune response to harmful agents, change of antioxidant systems and reduced production of interleukin-2, vascular failure, reduction of mast cell degranulation, and worsening of tissue oxygenation[61].
Diabetes contributes to a worse prognosis of an infection, with higher morbidity and mortality from sepsis compared to the general population[62]. Some studies on the biochemical characteristics of diabetic patients have found that the lymphocyte count is lower than in individuals without diabetes, while that of neutrophils is higher, in addition to a higher proportion of highly sensitive CRP and procalcitonin[63,64]. Inflammation-related biomarkers were also elevated in diabetics. All of these effects form an inflammatory storm, which leads to rapid deterioration in the framework of diseases like COVID-19[62].
Diabetes and associated complications can increase the risk of morbidity and mortality during acute infections due to the suppression of innate and humoral immune functions. Thus, diabetes is an important comorbidity to be considered in infectious processes, such as those triggered by COVID-19, especially when it accrues to other risk factors with inflammatory potential, including obesity. Both obesity and diabetes have deleterious effects on the host’s immunity, increasing the risk of infectious susceptibility and severity[62].
COVID-19
COVID-19 is an infectious disease caused by the SARS-CoV-2. SARS-CoV-2 is the third coronavirus identified to cause severe disease in humans. The first one was SARS-CoV, also from China, which caused a pandemic between 2002 and 2003. The second was the cause of the severe acute respiratory syndrome of the Middle East, called Middle East respiratory syndrome coronavirus (MERS-CoV), which was initially identified in the Arabian Peninsula in 2012[65]. Coronaviruses have the bat as their natural host and through recombination and genetic variation, adaptation and infection in other species was made possible. In the case of humans, possibly other mammals were the intermediate hosts, which is why COVID-19 is considered a zoonosis[66].
Coronaviruses have four structural proteins; spike (S), membrane (M), envelope (E) and nucleocapsid (N). Spike is composed of a trimetric glycoprotein transmembrane protruding from the viral surface, which determines the diversity of coronavirus and tropism in the host. The spike protein comprises two functional subunits: The S1 subunit is responsible for binding to the host cell receptor and the S2 subunit for fusion among viral and cellular membranes[67].
The SARS-CoV-2 infection is due to a highly transmissible virus among humans; the disease can be asymptomatic or oligosymptomatic, with a varied spectrum that can culminate in a condition of viral pneumonia, respiratory failure, involvement of other organs and systems, and death can be the outcome. The declared global pandemic, which affected 84233579 people and caused 1843293 deaths by the beginning of 2021, remains a challenge for public health, given its heterogeneity in the clinical conditions and the number of people affected[68].
The transmission of SARS-CoV-2 occurs directly between an infected and a susceptible individual by the inoculation of droplets expelled from the respiratory tract or saliva in the nasal, oral or conjunctiva mucosa during speech, coughing or sneezing. Transmission can also be indirect by sharing contaminated objects or surfaces or by aerosols[69]. The incubation time varies from 2 to 14 d, with an average of 5 d, in symptomatic cases. In the mild disease, the upper respiratory tract is usually affected, with the occurrence of fever, fatigue, myalgia, cough, odynophagia, nasal discharge and sneezing. There is a possibility of gastrointestinal symptoms, due to the presence of hemagglutinin esterase protein, with abdominal pain, vomiting and diarrhea. There are also many records of anosmia and ageusia as important symptoms in the diagnosis of suspected COVID-19[69].
In moderate disease, there is involvement of the lower respiratory tract, characterized by viral pneumonia, with fever and cough, and the pulmonary involvement is visualized by radiography or chest tomography. The presence or absence of hypoxia determines the severity criterion and the assistance to be provided. In cases where saturation is less than 92%, hospital monitoring with non-invasive oxygen supplementation is required. In some situations, failure to resolve hypoxia requires orotracheal intubation and monitoring in an intensive care unit[70].
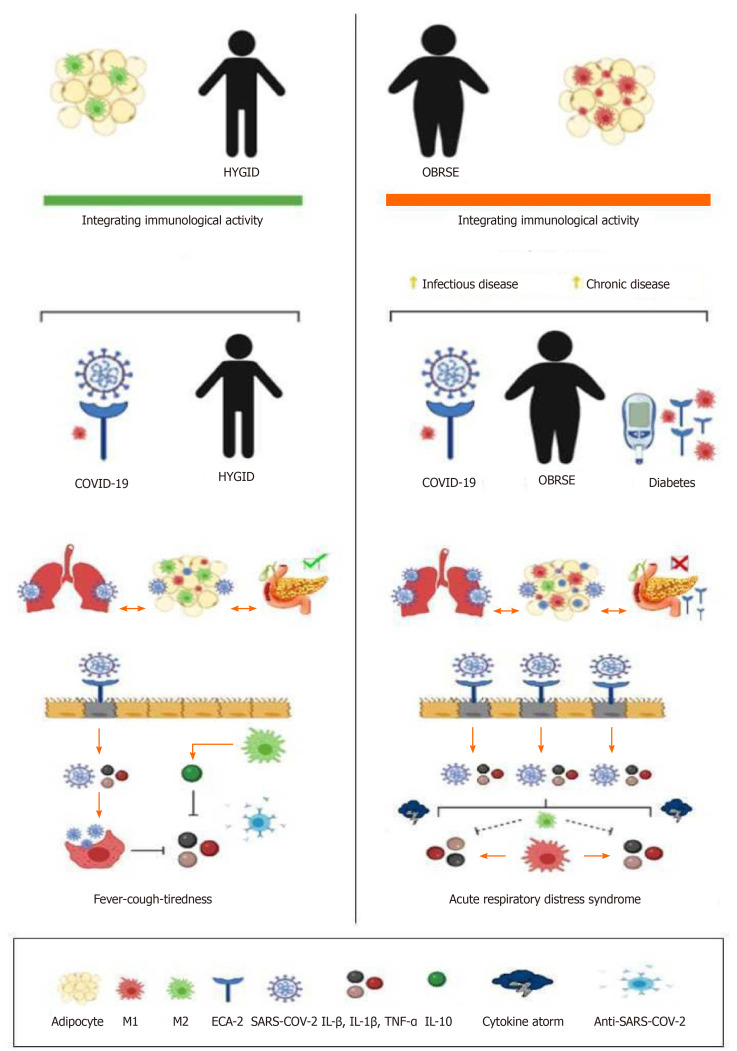
In the initial stage of infection, SARS-CoV-2 binds to target cells in the nasal tract, bronchial epithelial cells, or pneumocytes, by tying the spike (S) protein to the angiotensin converting enzyme-2 (ACE-2) receptor. The transmembrane serine protease type 2 (TMPRSS2), present in the host cell, causes the viral withdrawal through the cleavage of ACE-2 and activation of the SARS-CoV-2 protein S, which mediates the entry of the coronavirus into the host cells. ACE-2 and TMPRSS2 are present mainly in alveolar cells. After the viral adsorption, viral replication is activated. This process damages the host cell and causes proptosis due to damage caused by ATP, nucleic acids and ASC oligomers. The neighboring cells of the endothelium recognize cellular changes, initiating the signaling of the inflammatory process. The presence of alveolar macrophages and endothelial cells that trigger the generation of pro-inflammatory cytokines and chemokines (IL-6, interferon gamma (INTg)-induced protein 10, macrophage inflammatory protein-1 alpha (MIP-1α), MIP-1β, and mitochondrial pyruvate carrier-1), which attract macrophages, T cells, and INTγ creating a pro inflammatory feedback loop. In the production of neutralizing antibodies, the infection resolves; but there may be production of non-neutralizing antibodies generating the cytokine storm. This inadequate and exacerbated inflammatory response allows viremia to cause sepsis and shock, coagulation disorders, with damage to other target cells of vital organs such as the brain, heart and kidneys[70]. This inflammatory dysregulation, the main damage resulting from COVID-19, has been more common in patients with comorbidities and risk factors, probably due to low immunogenicity. In addition, diabetic and hypertensive patients have more ACE-2 receptors, as well as a greater number of these receptors in adipocytes due to the fact that obesity generates a low-grade inflammatory process[71-73] (Figure 1).
Figure 1.
Coronavirus disease 2019 physiological process in healthy and obese people. COVID-19: Coronavirus disease-2019; IL: Interleukin; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; TNF-α: Tumor necrosis factor-alpha.
The development of severe acute respiratory syndrome is seen more frequently in elderly patients with COVID-19 pneumonia and with underlying chronic diseases, such as systemic arterial hypertension, cardiovascular diseases, type 2 diabetes, chronic obstructive pulmonary disease or neoplasms[73]. All age groups are affected by COVID-19; however, individuals above 60 years of age present with the worst conditions, leading towards a death outcome[74].
DM, OBESITY, AND COVID-19
Infection by COVID-19 has highly impacted daily clinical practice, since practically all organs and biological systems suffer from this new infection, either because the virus directly affects specific tissues or because of indirect effects[75]. Due to the high prevalence of diabetes and obesity worldwide, it is important to understand aspects of coronavirus infection and the impact of multiple epidemics co-existence on health systems, as pre-existing conditions contribute to a worse prognosis, due to the immune system impairment[75].
DIABETES AND COVID-19
During previous SARS pandemics, the rate of admission to the intensive care units (ICUs), the need for mechanical ventilation and the mortality of diabetic patients was 3.1 times higher than that of non-diabetic patients[76]. During the 2012 (MERS-CoV) outbreak, diabetes was prevalent in almost 50% of the population and the odds ratio (OR) for severe or critical MERS-CoV ranged from 7.2 to 15.7 in the cohort of individuals with DM compared to the general population[76].
Recent studies have also shown a relationship between SARS-CoV-2 infection and the presence of diabetes: 34% to 58% COVID-19 patients had diabetes. Other reports suggest that patients with both COVID-19 and diabetes are more often associated with severe or critical cases. In a study with 138 patients with COVID-19, Wang et al[77] observed that 72% of patients with comorbidities, including diabetes, required admission to the ICU, compared with 37% of patients without comorbidities. Wu et al[78] evaluated 201 patients with COVID-19 and found that diabetic patients had a risk ratio of 2.34 (95% confidence interval [CI]: 1.35 to 4.05; P = 0.002) for acute respiratory syndrome. Diabetic patients usually exhibit a worse infection prognosis, with higher sepsis morbidity and mortality compared to the general population[79].
In addition to the presence of diabetes, the individual's hyperglycemic state has been associated with greater severity of COVID-19 and unfavorable outcome in infected patients, especially when micro and macrovascular complications are present[80]. In addition, SARS-CoV-2 infection has been associated to the onset of hyperglycemia, and the need to use insulin, especially in patients requiring ICU admission[81]. Diagnosis of diabetes can occur on admission and in a frequency similar to that of already known cases (about 15%), and can be a greater risk factor for severity of COVID-19 than pre-existing disease[77-81].
In addition, some medications used to treat COVID-19 patients can alter glycemic homeostasis in different ways, suggesting that infected patients are more susceptible to the worsening of the disease. However, there is still insufficient evidence to state that COVID-19 can also accelerate the onset of diabetes complications in diabetic patients who have been infected[80].
Adequate glycemic control is essential to reduce the severity of the clinical condition of infected patients. Considering poor control when glycated hemoglobin (HbA1c) levels were greater than 8%, a study of 80 patients with type II diabetes in India showed that patients with worse glycemic control had: Severe clinical manifestation of COVID-19, such as cough and shortage of breath, more severe inflammatory responses with high serum levels contents of fibrin degradation products, indicating hypercoagulability, D-dimer and interleukin IL-6, in addition to greater pulmonary impairment found in radiographic findings. Still, those patients needed more frequently intensive treatment, non-invasive ventilation, had longer hospitalization and higher mortality[82]. Such conditions had already been described in patients with diabetes during the Influenza pandemic in 2009. HbA1c levels > 9% were associated with a 60% increased risk of hospitalization and pneumonia-related severity during infection[82].
Thus, patients with diabetes are usually affected by a low-grade chronic inflammation, which facilitates cytokine storms, contributing to the serious results of COVID-19 and possible death. Still, one of the main risk factors in diabetic patients with severe condition is obesity[83].
OBESITY AND COVID-19
Considering that obese individuals also have a pre-defined pro-inflammatory medium, it is possible that COVID-19 may further exacerbate the inflammation, exposing them to higher levels of circulating inflammatory molecules compared to non-obese individuals[84]. In addition, it has been proven that the lung function is affected in several ways, in connection with the mechanical and inflammatory aspects, making obese people more likely to suffer from respiratory problems, symptoms and progression to respiratory failure[85].
In the current pandemic, there are higher rates of obesity and severe obesity in patients with COVID-19[86]. In the United States, among patients with COVID-19 under 60 years of age, those with 30 to 35 kg/m2 body mass index (BMI) and those with BMI above 35 kg/m2 were 1.8 and 3.6 times more likely to be admitted to the ICU, respectively, compared to those with adequate BMI[87]. In the United Kingdom, 38% of patients with COVID-19 admitted to the ICU were obese[88]. In China, where the first cases of COVID-19 occurred, investigations have shown that obesity increases approximately three times the risk of severe manifestation of the disease, with a consequent longer hospital stay[89].
The causal relationship between obesity and diabetes was suggested in 1965 in a longitudinal study carried out by the National Institute of Health in Arizona, where the risk of developing diabetes was 40-fold in obese women compared to those who remained slim[90]. From the first epidemiological reports, obesity and diabetes were identified as risk comorbidities for the development of more severe clinical forms of SARS-CoV-2 infection, since the cytokine storm can be amplified, leading to multisystemic failure and death[91,92].
Although the connection between these comorbidities is still not fully understood, in an obese and diabetic condition, the adipose tissue is impaired and may be directly or indirectly involved in interactions with SARS-CoV-2 at different levels, since the virus can change the fate of the adipocytes in adipose tissue or adipocyte-like cells in the lungs, which can positively regulate the genes associated with lipid metabolism in lung epithelial cells and the genes involved in leptin regulation[93,94].
Increased release of non-steroidal fatty acids is seen in both obesity and DM2 and both are associated with insulin resistance[95]. Insulin promotes glucose uptake and the increase of adipocytes, causing activation and recruitment of resident macrophages in adipose tissue and resulting in the release of pro-inflammatory cytokines and chemokines such as IL-1, IL-6, IL-8, monocyte-1 chemoattractive protein, and CRP[96,97]. The serum levels of IL-6 and TNF-α, therefore, are independent and significant predictors of the severity of the disease and deaths of COVID-19 patients[97]. These factors can increase the probability of developing endothelial dysfunction and platelet aggregation, causing the formation of occlusive thrombus in the heart and lungs in patients with COVID-19[98].
A tentative explanation for this relationship may be the fact that the expression of ACE-2, the functional receptor for SARS-CoV, is positively regulated in patients with obesity and diabetes[94], in addition to the deregulation of lipid synthesis and clearance, which can initiate or worsen lung inflammation and injury[93]. The presence of ACE-2 may allow SARS-CoV-2 to promote the adipocytes, which makes adipose tissue an important viral reservoir[94]. Consequently, adipose tissue can also be infected by SARS-CoV-2 and allow dissemination to other organs[99].
Cytokine production is a key element of the inflammatory and immunological response to viral infection, since after the host is released, the virus is first recognized by the innate immune system through molecular pattern recognition receptors, such as the receptors of C-type lectin and the NOD receptor of the toll-like receptor and exposure to the virus causes the expression of inflammatory factors in several ways, in particular the maturation of dendritic cells and the synthesis of interferons which role is to limit the spread of the virus and to accelerate phagocytosis of viral antigens[100].
In individuals with obesity and diabetes, the low-grade chronic inflammatory state can worsen the inflammatory response to SARS-CoV-2[101-103] infection. The prevalence of viral infection and its clinical consequences are more evident in the elderly and in individuals with obesity and diabetes, since this group exhibits weak immune activity and increased oxidative stress due to the decline in endogenous antioxidants[104].
Obesity and DM have been considered critical risk factors for different infections, post-infection complications and mortality from serious infections. Both have deleterious effects on the host's immunity, mainly increasing the risk of infectious susceptibility and disease severity[105].
Studies have shown obesity and type 2 diabetes as comorbidities in the development of SARS into COVID-19, in addition to being associated with severe forms of COVID-19 in all ethnic groups[105,106]. In a study carried out in the United States with 5700 hospitalized patients with severe forms of COVID-19, the authors observed that many of them presented with obesity (41%) or type 2 diabetes (33%). A retrospective study of 1158 hospitalized patients in Kuwait revealed that patients with morbid obesity and type 2 diabetes were much more likely to be admitted to the ICU, showing an OR of 5.18 and 9.38, respectively[106].
The WHO has been publishing a series of documents and guidelines to facilitate the understanding of the population with diabetes and obesity, and to direct them to self-care in times of quarantine and social isolation[107]. The recommendations continue to be related to health care in general: Healthy eating, practice physical activity, monitor and control glycemic rates and indexes, take medications correctly and reduce the risk of contagion[107].
When it comes to diseases that are largely related directly to modifiable factors, such as obesity and type 2 diabetes, it is important for the population to assume self-care. In this connection, recommendations and access to information on the part of patients, as well as the guidance of health professionals involved in the treatment of those patients is essential. It is necessary that patients become protagonists during treatment, have autonomy and enhance disease control.
CONCLUSION
The large increase in cases of COVID-19 infection worldwide in people with obesity and DM stands out as a factor of concern for public health. These comorbidities accelerate the inflammatory process and post-infection complications, such as increased production of cytokines, which when associated with the consequences of SARS-CoV-2 infection produce harmful effects, which can lead to death.
SARS-CoV-2 infection can increase the risk of sepsis in people with obesity and DM, especially for sedentary or insufficiently active people, as they have low respiratory and neuromuscular capacity. This risk is further exacerbated by the increase in circulating inflammatory molecules in individuals with obesity, and the diabetogenic effect that drugs for the treatment of SARS-CoV-2 can produce.
Therefore, it is recommended to patients with these comorbidities self-care such as the practice of regular physical activity, balanced diet and, in the case of individuals with DM, glycemic control. Social isolation to avoid contamination, maintenance of a healthy lifestyle, are still the best forms of prevention, as long it is not possible to immunize everyone against SARS-CoV-2. Among post-COVID patients, rehabilitation with follow-up for lifestyle changes is essential to improve quality of life and to avoid hospital readmissions.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: January 20, 2021
First decision: May 3, 2021
Article in press: June 4, 2021
Specialty type: Health care sciences and services
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng X S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
Contributor Information
Adriano Alberti, Research Group in Neurodevelopment of Childhood and Adolescence, Laboratory of Experimental Neuroscience, Postgraduate Program in Health Sciences, University of South Santa Catarina (Unisul), Palhoça, 88137-270, Santa Catarina, Brazil. adrianoalberti90@hotmail.com.
Fabiana Schuelter-Trevisol, Brazil Clinical Research Center, Postgraduate Program in Health Sciences, University of Southern Santa Catarina at Tubarão, Tubarão, 88704 -900, Santa Catarina, Brazil.
Betine Pinto Moehlecke Iser, Brazil Clinical Research Center, Postgraduate Program in Health Sciences, University of Southern Santa Catarina at Tubarão, Tubarão, 88704 -900, Santa Catarina, Brazil.
Eliane Traebert, Postgraduate Programme in Health Sciences, University of Southern Santa Catarina, Palhoça, 88137-270, Santa Catarina, Brazil.
Viviane Freiberger, Research Group in Neurodevelopment of Childhood and Adolescence, Laboratory of Experimental Neuroscience, Postgraduate Program in Health Sciences, University of South Santa Catarina (Unisul), Palhoça, 88137-270, Santa Catarina, Brazil.
Leticia Ventura, Research Group in Neurodevelopment of Childhood and Adolescence, Laboratory of Experimental Neuroscience, Postgraduate Program in Health Sciences, University of South Santa Catarina (Unisul), Palhoça, 88137-270, Santa Catarina, Brazil.
Gislaine Tezza Rezin, Brazil Clinical Research Center, Postgraduate Program in Health Sciences, University of Southern Santa Catarina at Tubarão, Tubarão, 88704 -900, Santa Catarina, Brazil.
Bruna Becker da Silva, Postgraduate Programme in Health Sciences, University of Southern Santa Catarina, Palhoça, 88137-270, Santa Catarina, Brazil.
Fabiana Meneghetti Dallacosta, Postgraduate Program in Biosciences and Health, University of the West of Santa Catarina, Joaçaba, 89600-000, Santa Catarina, Brazil.
Leoberto Grigollo, Research Group in Neurodevelopment of Childhood and Adolescence, Laboratory of Experimental Neuroscience, Postgraduate Program in Health Sciences, University of South Santa Catarina (Unisul), Palhoça, 88137-270, Santa Catarina, Brazil.
Paula Dias, Research Group in Neurodevelopment of Childhood and Adolescence, Laboratory of Experimental Neuroscience, Postgraduate Program in Health Sciences, University of South Santa Catarina (Unisul), Palhoça, 88137-270, Santa Catarina, Brazil.
Gracielle Fin, Department of Physical Education, University of the West of Santa Catarina, Joaçaba, 89600-000, Santa Catarina, Brazil.
Josiane Aparecida De Jesus, Postgraduate Program in Biosciences and Health, University of the West of Santa Catarina, Joaçaba, 89600-000, Santa Catarina, Brazil.
Fabiane Pertille, Postgraduate Program in Biosciences and Health, University of the West of Santa Catarina, Joaçaba, 89600-000, Santa Catarina, Brazil.
Carina Rossoni, Environmental Health Institute of the Faculty of Medicine of the University of Lisbon, Lisboa, 1649-029, Portugal.
Ben Hur Soares, Health Science, University of Passo Fundo, Passo Fundo, 99052-900, Rio Grande do Sul, Brazil.
Rudy José Nodari Júnior, Research department, Salus Dermatoglifia, Luzerna , 89609-000, Santa Catarina, Brazil.
Clarissa Martinelli Comim, Research Group in Neurodevelopment of Childhood and Adolescence, Laboratory of Experimental Neuroscience, Postgraduate Program in Health Sciences, University of South Santa Catarina (Unisul), Palhoça, 88137-270, Santa Catarina, Brazil.
References
- 1.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care . 2016;22:s176–s185. [PubMed] [Google Scholar]
- 2.Vedrova IN, Sapelkina IM. [The effect of various doses of vitamin A on the rat epidermis] Vestn Dermatol Venerol . 1968;42:35–38. [PubMed] [Google Scholar]
- 3.Bradbury EM. Conformations and flexibilities of histones and high mobility group (HMG) proteins in chromatin structure and function. Ciba Found Symp . 1983;93:246–270. doi: 10.1002/9780470720752.ch14. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National diabetes statistics report, 2020. [cited 4 May 2021]. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html .
- 5.Nazir MA, AlGhamdi L, AlKadi M, AlBeajan N, AlRashoudi L, AlHussan M. The burden of Diabetes, Its Oral Complications and Their Prevention and Management. Open Access Maced J Med Sci . 2018;6:1545–1553. doi: 10.3889/oamjms.2018.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahara T, Saeki M, Nosaka S, Shimoyamada K, Suemitsu I, Nakajima Y, Yoshikawa T, Ishikawa T. [The use of high concentration ferric ammonium citrate (FAC) solution as a negative bowel contrast agent: application in MR cholangiography] Nihon Igaku Hoshasen Gakkai Zasshi . 1995;55:697–699. [PubMed] [Google Scholar]
- 7.Ruigrok TJ, Cella F, Voogd J. Connections of the lateral reticular nucleus to the lateral vestibular nucleus in the rat. An anterograde tracing study with Phaseolus vulgaris leucoagglutinin. Eur J Neurosci . 1995;7:1410–1413. doi: 10.1111/j.1460-9568.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 8.Favilli S, Iacopetti L, Fradella GA, Righi D, Nuzzaci G. Observations on changes in intermittent claudication after the administration of creatine phosphate. Pharmatherapeutica . 1982;3:221–226. [PubMed] [Google Scholar]
- 9.The International Diabetes Federation. Type 2 Diabetes. IDF 2020. [cited 4 May 2021]. Available from: https://www.idf.org/aboutdiabetes/type-2-diabetes.html .
- 10.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. [cited 4 May 2021]. Available from: https://www.who.int/dg/ speeches/detail/who-director-general-s-openingremarks-at-the-media-briefing-on-covid-19---11- march-2020.
- 11.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet . 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 12.Azziz R, Deal CL, Potter HD, Gargosky SE, Rosenfeld RG. Regulation of extragonadal insulin-like growth factor-binding protein-3 by testosterone in oophorectomized women. J Clin Endocrinol Metab . 1994;79:1747–1751. doi: 10.1210/jcem.79.6.7527410. [DOI] [PubMed] [Google Scholar]
- 13.Armanini MP, McMahon SB, Sutherland J, Shelton DL, Phillips HS. Truncated and catalytic isoforms of trkB are co-expressed in neurons of rat and mouse CNS. Eur J Neurosci . 1995;7:1403–1409. doi: 10.1111/j.1460-9568.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonomo L. [Antibiotic resistance: a serious danger too ignored] Recenti Prog Med . 1995;86:47. [PubMed] [Google Scholar]
- 15.World Health Organization. Global strategy on diet, physical activity and health: childhood overweight and obesity. [cited 4 May 2021]. Available from: https://www.who.int/dietphysicalactivity/strategy/eb11344/strategy_english_web.pdf .
- 16.Hill PJ. Reversals in reading: are they abnormal? Am J Optom Physiol Opt . 1980;57:162–165. [PubMed] [Google Scholar]
- 17.Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Pediatr Diabetes . 2019;20:5–9. doi: 10.1111/pedi.12787. [DOI] [PubMed] [Google Scholar]
- 18.Kunishima S, Mizuno R, Fujishiro N, Ohno R. [A case of chronic neutrophilic leukemia with abnormal karyotype] Rinsho Byori . 1989;37:943–947. [PubMed] [Google Scholar]
- 19.Harrington MG, McGeorge AP, Ballantyne JP, Beastall G. A prospective survey for insulinomas in a neurology department. Lancet . 1983;1:1094–1095. doi: 10.1016/s0140-6736(83)91923-2. [DOI] [PubMed] [Google Scholar]
- 20.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism . 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA . 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Adult Obesity Prevalence Maps. [cited 4 May 2021]. Available from: https://www.cdc.gov/obesity/data/prevalence-maps.html .
- 23.World Health Organization. Obesity and overweight. [cited 4 May 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight .
- 24.Patton GC, Coffey C, Carlin JB, Sawyer SM, Williams J, Olsson CA, Wake M. Overweight and obesity between adolescence and young adulthood: a 10-year prospective cohort study. J Adolesc Health . 2011;48:275–280. doi: 10.1016/j.jadohealth.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Alberti A, Ruiz Reyes MA, De Jesus JA, Rossoni C, Grigollo L, Da Silva BB, Fin G, Baretta E, Comim CM, Nodari RJ Jr. Identification of obesity in children and teenagers. Minerva Pediatr . 2021 doi: 10.23736/S2724-5276.20.05731-X. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev . 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord . 2018;23:149–157. doi: 10.1007/s40519-018-0481-6. [DOI] [PubMed] [Google Scholar]
- 28.Osborn R, Boland T, DeLuchi S, Beirne OR. Osteomyelitis of the mandible in a patient with malignant osteopetrosis. J Oral Med . 1985;40:76–80. [PubMed] [Google Scholar]
- 29.Badimon L, Cubedo J. Adipose tissue depots and inflammation: effects on plasticity and resident mesenchymal stem cell function. Cardiovasc Res . 2017;113:1064–1073. doi: 10.1093/cvr/cvx096. [DOI] [PubMed] [Google Scholar]
- 30.Michailidou Z. Fundamental roles for hypoxia signalling in adipose tissue metabolism and inflammation in obesity. Curr Opin Physiol . 2019;12:39–43. [Google Scholar]
- 31.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev . 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 32.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol . 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 33.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol . 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol . 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Bollinger T, Gies S, Naujoks J, Feldhoff L, Bollinger A, Solbach W, Rupp J. HIF-1α- and hypoxia-dependent immune responses in human CD4+CD25high T cells and T helper 17 cells. J Leukoc Biol . 2014;96:305–312. doi: 10.1189/jlb.3A0813-426RR. [DOI] [PubMed] [Google Scholar]
- 36.Westendorf AM, Skibbe K, Adamczyk A, Buer J, Geffers R, Hansen W, Pastille E, Jendrossek V. Hypoxia Enhances Immunosuppression by Inhibiting CD4+ Effector T Cell Function and Promoting Treg Activity. Cell Physiol Biochem . 2017;41:1271–1284. doi: 10.1159/000464429. [DOI] [PubMed] [Google Scholar]
- 37.Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes . 2020;10:e12365. doi: 10.1111/cob.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel PS, Buras ED, Balasubramanyam A. The role of the immune system in obesity and insulin resistance. J Obes . 2013;2013:616193. doi: 10.1155/2013/616193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol . 2015;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol . 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopategi A, López-Vicario C, Alcaraz-Quiles J, García-Alonso V, Rius B, Titos E, Clària J. Role of bioactive lipid mediators in obese adipose tissue inflammation and endocrine dysfunction. Mol Cell Endocrinol . 2016;419:44–59. doi: 10.1016/j.mce.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol . 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 43.Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest . 2018;48:1–26. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- 44.de Mello AH, Schraiber RB, Goldim MPS, Mathias K, Mendes C, Corrêa MEAB, Gomes ML, Silveira PCL, Schuck PF, Petronilho F, Rezin GT. Omega-3 polyunsaturated fatty acids have beneficial effects on visceral fat in diet-induced obesity model. Biochem Cell Biol . 2019;97:693–701. doi: 10.1139/bcb-2018-0361. [DOI] [PubMed] [Google Scholar]
- 45.Cancello R, Clément K. Is obesity an inflammatory illness? BJOG . 2006;113:1141–1147. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 46.de Mello AH, Uberti MF, de Farias BX, de Souza NAR, Rezin GT. n-3 PUFA and obesity: from peripheral tissues to the central nervous system. Br J Nutr . 2018;119:1312–1323. doi: 10.1017/S0007114518000429. [DOI] [PubMed] [Google Scholar]
- 47.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell . 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol . 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petronilho F, Giustina AD, Nascimento DZ, Zarbato GF, Vieira AA, Florentino D, Danielski LG, Goldim MP, Rezin GT, Barichello T. Obesity Exacerbates Sepsis-Induced Oxidative Damage in Organs. Inflammation . 2016;39:2062–2071. doi: 10.1007/s10753-016-0444-x. [DOI] [PubMed] [Google Scholar]
- 50.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab . 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shefer G, Marcus Y, Stern N. Is obesity a brain disease? Neurosci Biobehav Rev . 2013;37:2489–2503. doi: 10.1016/j.neubiorev.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Mazon JN, de Mello AH, Ferreira GK, Rezin GT. The impact of obesity on neurodegenerative diseases. Life Sci . 2017;182:22–28. doi: 10.1016/j.lfs.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 53.de Bona Schraiber R, de Mello AH, Garcez ML, de Bem Silveira G, Zacaron RP, de Souza Goldim MP, Budni J, Silveira PCL, Petronilho F, Ferreira GK, Rezin GT. Diet-induced obesity causes hypothalamic neurochemistry alterations in Swiss mice. Metab Brain Dis . 2019;34:565–573. doi: 10.1007/s11011-018-0337-9. [DOI] [PubMed] [Google Scholar]
- 54.Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, Mirhafez SR, Moohebati M, Esmaily H, Ghazavi H, Saberi Karimian M, Parizadeh SM, Mohammadi M, Mohaddes Ardabili H, Ferns GA, Ghayour-Mobarhan M. Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J Clin Lab Anal . 2016;30:672–676. doi: 10.1002/jcla.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.American Diabetes Association . (2) Classification and diagnosis of diabetes. Diabetes Care . 2015;38 Suppl:S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 56.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract . 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 57.Flor LS, Campos MR. The prevalence of diabetes mellitus and its associated factors in the Brazilian adult population: evidence from a population-based survey. Rev Bras Epidemiol . 2017;20:16–29. doi: 10.1590/1980-5497201700010002. [DOI] [PubMed] [Google Scholar]
- 58.Iser BP, Malta DC, Duncan BB, de Moura L, Vigo A, Schmidt MI. Prevalence, correlates, and description of self-reported diabetes in brazilian capitals - results from a telephone survey. PLoS One . 2014;9:e108044. doi: 10.1371/journal.pone.0108044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malta DC, Duncan BB, Schmidt MI, Machado ÍE, Silva AGD, Bernal RTI, Pereira CA, Damacena GN, Stopa SR, Rosenfeld LG, Szwarcwald CL. Prevalence of diabetes mellitus as determined by glycated hemoglobin in the Brazilian adult population, National Health Survey. Rev Bras Epidemiol 2019; 22Suppl 02: E190006.SUPL. 2 doi: 10.1590/1980-549720190006.supl.2. [DOI] [PubMed] [Google Scholar]
- 60.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet . 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao S, Lau A, So HC. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of SARS-CoV-2: A Mendelian Randomization Analysis Highlights Tentative Relevance of Diabetes-Related Traits. Diabetes Care . 2020;43:1416–1426. doi: 10.2337/dc20-0643. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19) Diabetes Metab Res Rev . 2021;37:e3377. doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev . 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, Yu X, Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care . 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect . 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fung M, Otani I, Pham M, Babik J. Zoonotic coronavirus epidemics: Severe acute respiratory syndrome, Middle East respiratory syndrome, and coronavirus disease 2019. Ann Allergy Asthma Immunol . 2021;126:321–337. doi: 10.1016/j.anai.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mariano G, Farthing RJ, Lale-Farjat SLM, Bergeron JRC. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front Mol Biosci . 2020;7:605236. doi: 10.3389/fmolb.2020.605236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Health Organization. Coronavirus disease (COVID-19) pandemic. Dashboard in 5 January 2021. [cited 4 May 2021]. Available from: https://covid19.who.int/
- 69.Iser BPM, Sliva I, Raymundo VT, Poleto MB, Schuelter-Trevisol F, Bobinski F. Suspected COVID-19 case definition: a narrative review of the most frequent signs and symptoms among confirmed cases. Epidemiol Serv Saude . 2020;29:e2020233. doi: 10.5123/S1679-49742020000300018. [DOI] [PubMed] [Google Scholar]
- 70.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol . 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.World Health Organization. Emergencies. Diseases. Coronavirus disease 2019 (COVID-2019) pandemic. [cited 4 May 2021]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 .
- 72.Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses . 2019;11 doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA . 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuelter-Trevisol F, Raimundo LJ, Soccas HD, Antunes AF, Mohr RLD, Marcon CEM, Trevisol DJ. Assessment of patients with Covid-19 hospitalized in southern Santa Catarina. Rev Soc Bras Med Trop . 2020;53:e20200579. doi: 10.1590/0037-8682-0579-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marazuela M, Giustina A, Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord . 2020;21:495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA . 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 77.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA . 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med . 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet . 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol . 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract . 2020;162:108142. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhandari S, Rankawat G, Singh A, Gupta V, Kakkar S. Impact of glycemic control in diabetes mellitus on management of COVID-19 infection. Int J Diabetes Dev Ctries . 2020:1–6. doi: 10.1007/s13410-020-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol . 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol . 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 85.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med . 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Sabah S, Al-Haddad M, Al-Youha S, Jamal M, Almazeedi S. COVID-19: Impact of obesity and diabetes on disease severity. Clin Obes . 2020;10:1–6. doi: 10.1111/cob.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, Stachel A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis . 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomson RJ, Hunter J, Dutton J, Schneider J, Khosravi M, Casement A, Dhadwal K, Martin D. Clinical characteristics and outcomes of critically ill patients with COVID-19 admitted to an intensive care unit in London: A prospective observational cohort study. PLoS One . 2020;15:e0243710. doi: 10.1371/journal.pone.0243710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J, Zheng MH. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care . 2020;43:e72–e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 90.Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, Speizer FE. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol . 1990;132:501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 91.Fernández García L, Puentes Gutiérrez AB, García Bascones M. [Relationship between obesity, diabetes and ICU admission in COVID-19 patients] Med Clin (Barc) . 2020;155:314–315. doi: 10.1016/j.medcle.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smati S, Tramunt B, Wargny M, Caussy C, Gaborit B, Vatier C, Vergès B, Ancelle D, Amadou C, Bachir LA, Bourron O, Coffin-Boutreux C, Barraud S, Dorange A, Fremy B, Gautier JF, Germain N, Larger E, Laugier-Robiolle S, Meyer L, Monier A, Moura I, Potier L, Sabbah N, Seret-Bégué D, Winiszewski P, Pichelin M, Saulnier PJ, Hadjadj S, Cariou B, Gourdy P CORONADO investigators. Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: Results from the CORONADO study. Diabetes Obes Metab . 2021;23:391–403. doi: 10.1111/dom.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drucker DJ. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr Rev . 2020;41 doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kruglikov IL, Scherer PE. The Role of Adipocytes and Adipocyte-Like Cells in the Severity of COVID-19 Infections. Obesity (Silver Spring) . 2020;28:1187–1190. doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol . 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalez LL, Garrie K, Turner MD. Type 2 diabetes - An autoinflammatory disease driven by metabolic stress. Biochim Biophys Acta Mol Basis Dis . 2018;1864:3805–3823. doi: 10.1016/j.bbadis.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 97.Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin Chim Acta . 2019;496:35–44. doi: 10.1016/j.cca.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 98.Del Valle DM, Kim-Schulze S, Hsin-Hui H, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz T, Madduri D, Stock A, Marron T, Xie H, Patel MK, van Oekelen O, Rahman A, Kovatch P, Aberg J, Schadt E, Jagannath S, Mazumdar M, Charney A, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature helps predict COVID-19 severity and death. medRxiv . 2020 doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bourgeois C, Gorwood J, Barrail-Tran A, Lagathu C, Capeau J, Desjardins D, Le Grand R, Damouche A, Béréziat V, Lambotte O. Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Front Microbiol . 2019;10:2837. doi: 10.3389/fmicb.2019.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci . 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med . 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration. UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet . 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cardinali DP, Hardeland R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology . 2017;104:382–397. doi: 10.1159/000446543. [DOI] [PubMed] [Google Scholar]
- 105.Frydrych LM, Bian G, O'Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol . 2018;104:525–534. doi: 10.1002/JLB.5VMR0118-021RR. [DOI] [PubMed] [Google Scholar]
- 106.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA . 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.World Health Organization. Physical inactivity a leading cause of disease and disability, warns WHO. 2020. COVID-19: vulnerable and high risk groups. 2020. [cited 4 May 2021]. Available from: https://www.who.int/westernpacific/emergencies/covid-19/information/high-risk-groups .