Figure 2.
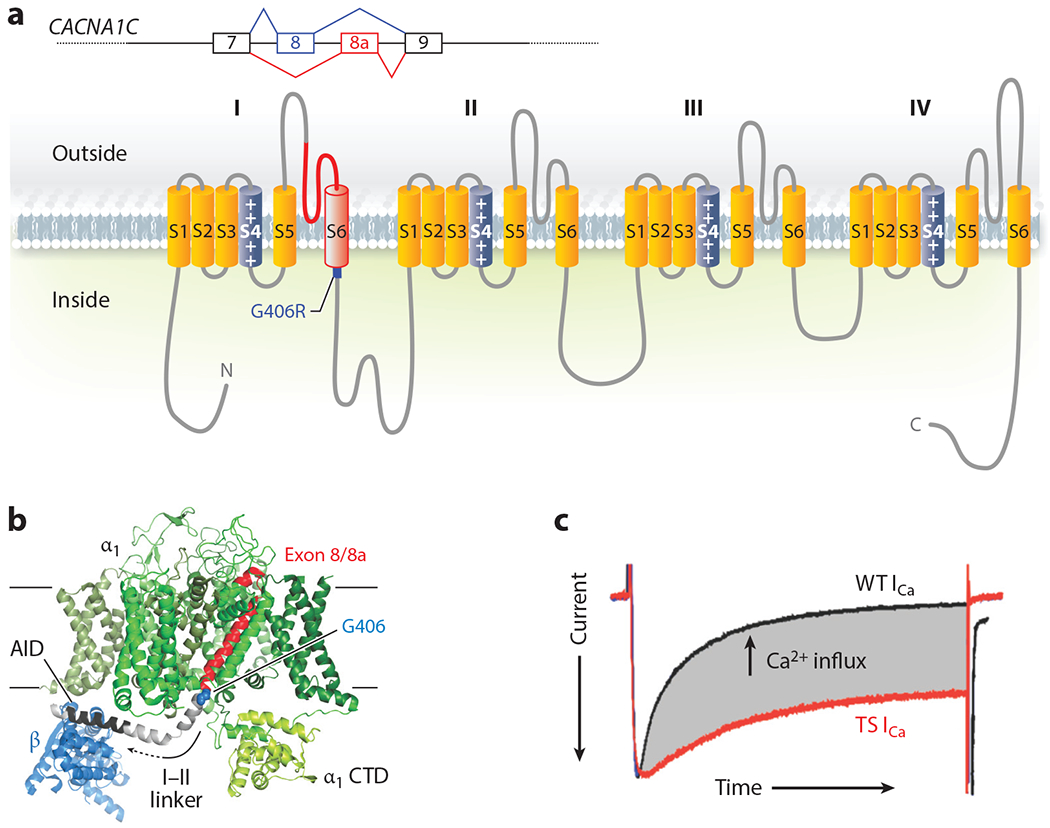
Structure and function of Timothy syndrome (TS) mutation. (a) Schematic of the alternative splicing event for exon 8 or 8a in CACNA1C and the exon in the context of the CaV1.2 α1C subunit. (b) Structure of the homologous rabbit CaV1.1 (Protein Data Bank: 5GVY) shown from the membrane. Only the α1 subunit and the β subunit (blue) are shown. The individual α1 domains (DI–DIV) are colored in various shades of green except for the polypeptide encoded by exon 8 (or exon 8a) in red, with the G406 residue that is mutated to G406R highlighted in blue; the linker between domain I and domain II (I–II linker) is depicted in gray, in which the α interaction domain (AID) is highlighted in black. (c) Voltage-clamp Ca2+ current recording from a cardiomyocyte isolated from a mouse with the TS mutation (red) overlaid with a recording from a cardiomyocyte isolated from a wild-type (WT) littermate. The currents are scaled for comparison, and the enhanced Ca2+ influx through the TS mutant channel is highlighted in gray (E.Q. Wei and G.S. Pitt, unpublished data).
