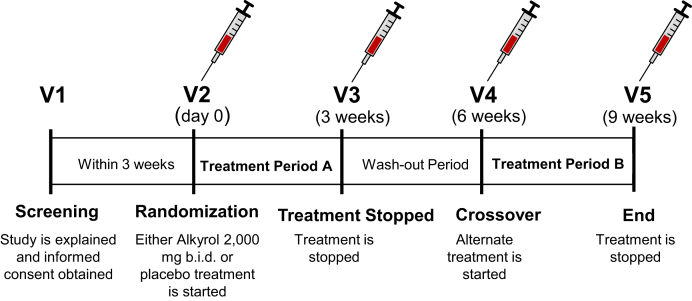
Fig. 3.
Study design for shark liver oil supplementation in humans. Participants were recruited into the study and asked to attend an initial screening. At the screening visit, participants underwent a medical examination to assess their eligibility. Eligible participants were recalled, within three weeks, where they were randomized to take either Alkyrol® (shark liver oil gel caps) or placebo for three weeks. At the three-week visit, the participants discontinued the treatment/placebo for a three-week washout period. At visit 4, the participants commenced the alternative treatment for 3 weeks. At visit 5, the participants underwent the same medical examination as visit 1 to assess any change throughout the study period. Fasting blood samples from each participant were collected at the initial screening and at the start and end of each intervention.