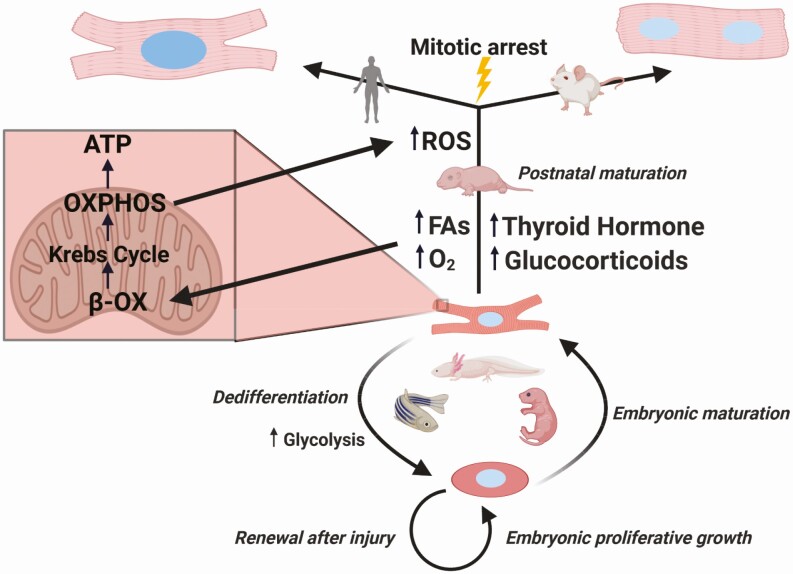
Figure 2.
Interactions of cardiomyocyte metabolism and the cell cycle across ontogeny and phylogeny. Immature cardiomyocytes proliferate rapidly during embryonic development. In organisms such as neonatal mice, zebrafish, and axolotls, cardiomyocytes can respond to injury by glycolysis-dependent reprogramming to a fetal-like state. This allows for repair by myocardium renewal. After birth, mammals rapidly undergo extensive metabolic maturation, stimulated by increased circulating fatty acids (FAs) and oxygen tension. Hormones such as thyroid hormone (TH) and glucocorticoids stimulate increased beta-oxidation (β-OX), which feeds the tricarboxylic acid cycle (Krebs cycle) and oxidative phosphorylation (OXPHOS); subsequent reactive oxygen species (ROS) surging induces the DNA damage response and arrests the cell cycle. Future cycling is primarily endoreduplicative, leading to polyploid cardiomyocytes that are primarily multinucleated in mice and multiploid mononucleated in humans. As a result, cardiomyocytes in the mature mammalian heart cannot mount a regenerative response to injury.