FIGURE 1.
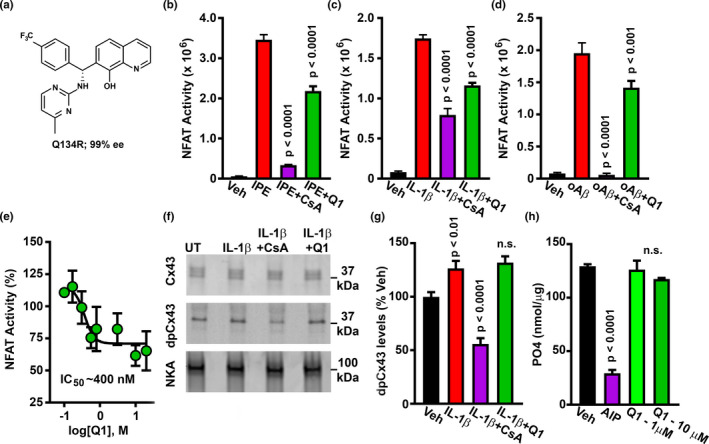
Q134R inhibits NFAT signaling but does not inhibit CN activity. (a) Chemical structure of Q134R. B‐D, Mean ± SEM NFAT‐dependent luciferase activity in primary astrocytes treated with the following CN/NFAT activators: 1 μM ionomycin and 1 μM phorbol ester (IPE) (b), 10 ng/ml IL‐1β (c), or 65 nM oligomeric Aβ peptides (d). CN/NFAT activators were also co‐delivered with the CN inhibitor, 5 μM CsA or 10 μM Q134R. Vehicle (Veh)‐treated cells served as a control. In each case, Q134R significantly inhibited NFAT‐luciferase expression. (e) Mean ± SEM NFAT‐luciferase expression (%) in primary astrocytes treated with 10 ng/ml IL‐1β in the presence of increasing Q134R concentrations. Q134R inhibited astrocytic NFAT signaling in a concentration‐dependent manner. (f) Representative western blot for total Cx43, dpCx43, and Na/K ATPAse loading control in primary astrocytes treated with Veh, or 10 ng/ml IL‐1β in the presence/absence of 5 μM CsA or 10 μM Q134R. Full blots are shown in Figure S2. (g) Mean ± SEM dpCx43 levels (% of Veh) under the conditions shown in panel f. CsA inhibited dephosphorylation of Cx43, but Q134R did not. (h) Mean ± SEM PO4 released from a synthetic phosphopeptide in response to in vitro CN activity. Dephosphorylation is inhibited by CN‐AIP (100 μM), but not by Q134R. For panels b‐e, n = six 35 mm dishes per condition. For panel h, n = 4 pooled samples per condition
