FIGURE 6.
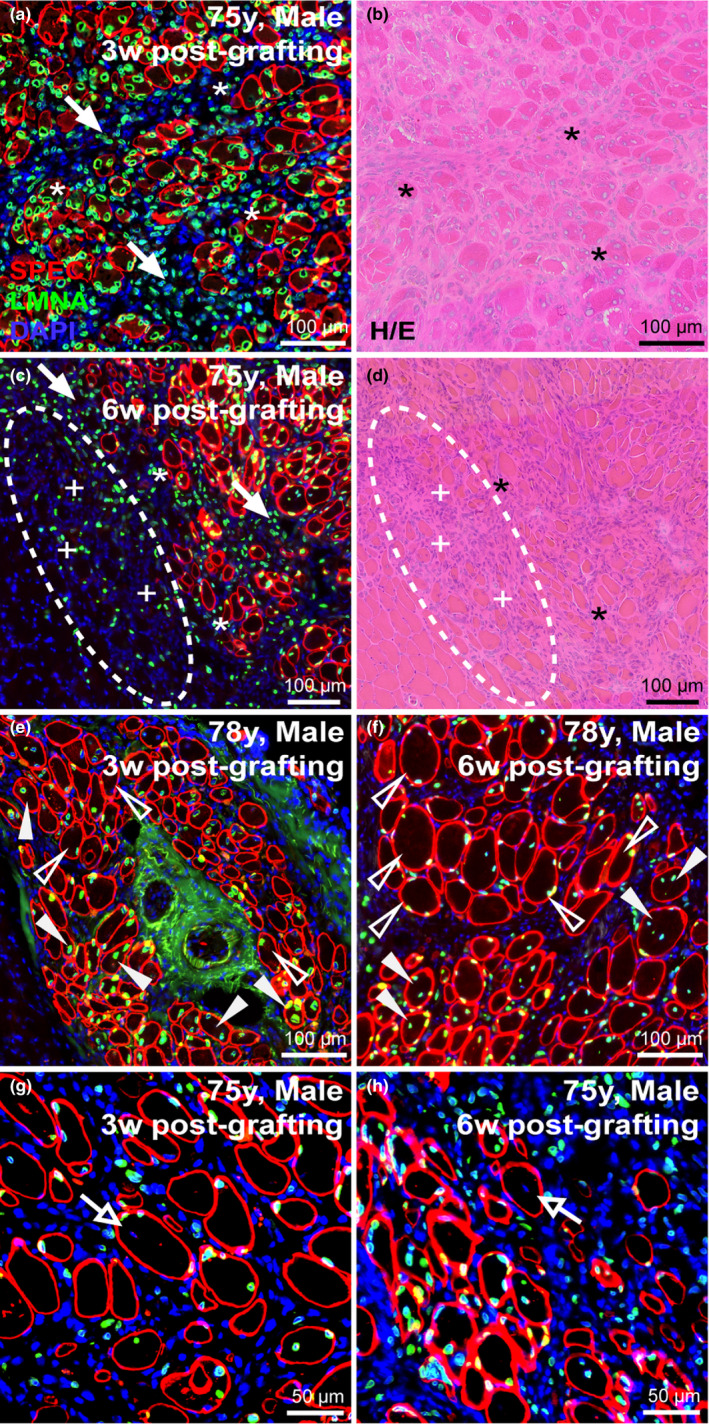
Myonuclear localization and migration post‐engraftment. (a‐d) Longitudinal assessment of 75‐year‐old sample harvested either 3‐week (a, b) or 6‐week (c, d) post‐engraftment assessed by immunostaining human‐specific protein (a, c) and H/E histology (c, d). Immunostaining for human‐specific spectrin and lamin A/C indicate frequency of central nucleation or peripheral nucleation in regenerated human myofibers 3‐week (a) or 6‐week (c) post‐engraftment. Prevalence of interstitial mononuclear cell types indicated at intervals post‐engraftment (solid arrows) and their increased presence beyond the boundary of mature human myofibers prevalent in samples 6‐week post‐engraftment (c, d; dotted circular region). Within these new regions low‐intensity spectrin membrane staining was observed in small‐diameter fibers (c, d; indicated by “+” signs adjacent to fibers). Asterisks (*) denote muscle orientation between serial cross‐sections (a‐d). (e, f) Longitudinal assessment of regeneration and nuclear localization after regeneration of the 78‐year‐old sample harvested either 3‐week (e) or 6‐week (f) post‐engraftment. Solid arrowheads indicate centrally nucleated fibers, while empty arrowheads indicate myonuclei that have adopted a peripheral position within the myofiber (e, f). (g, h) Myonuclei of mouse origin (negative for human lamin A/C) observed within regenerated human spectrin‐positive fibers; open arrows identify murine central myonuclei. Scale bars represent 100 μm (a‐f) or 50 μm (g, h).
