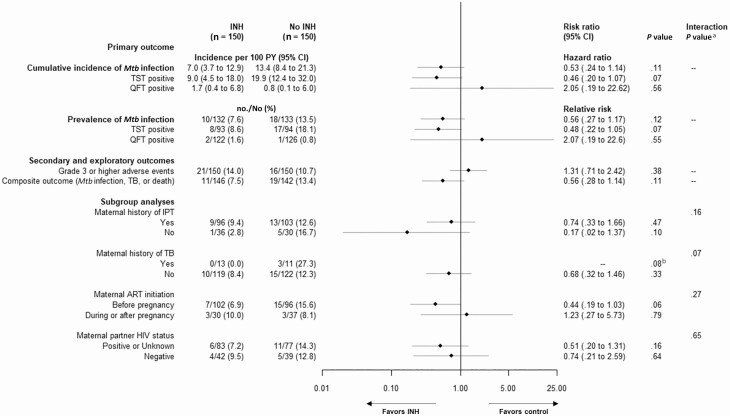
Figure 2.
Primary, secondary, and exploratory outcomes including subgroup analyses. The primary outcome is Mycobacterium tuberculosis (Mtb) infection (either tuberculin skin test or QuantiFERON-TB Gold Plus) 12 months after randomization utilizing a modified intention-to-treat approach including all participants who underwent randomization irrespective of receipt of trial medication with at least 1 measure of Mtb infection, excluding 2 children found to be human immunodeficiency virus DNA positive in the no isoniazid arm during the study. Exploratory composite outcome of Mtb infection, death, or tuberculosis includes all infants with known vital or tuberculosis status at the end of the trial. Subgroup analyses are based on baseline characteristics at the trial entry and include only participants with available primary outcome Mtb assessment. aInteraction between study group and subgroup. bBy Fisher exact test due to zero cases in the isoniazid arm. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; INH, isoniazid; IPT, isoniazid preventive therapy; Mtb, Mycobacterium tuberculosis; PY, person-years; QFT, QuantiFERON-TB Gold Plus; TB, tuberculosis; TST, tuberculin skin test.