Figure 1.
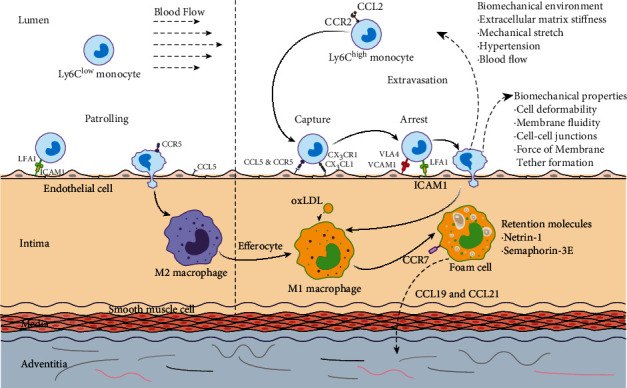
Cytokines, receptors, and biomechanical factors affecting the entry and egress of monocytes in the progression of atherosclerosis. The accumulation of Ly6Chigh monocytes in the nascent atherosclerotic plaque is triggered by the molecules secreted by endothelial cells, such as CCL2, CCL5, and CXC3L1. The interaction between lymphocyte function-associated antigen 1 (LFA1) or very late antigen 4 (VLA4) and endothelial adhesion molecules, such as intercellular adhesion molecule 1 (ICAM1) and vascular adhesion molecule 1 (VCAM1), facilitates the firm adhesion formation. After entering the intima, Ly6Chigh monocytes differentiate into M1-type macrophages that take up LDL and finally become foam cells. On the other hand, macrophages can be released from the plaque by the interaction of CCR7, CCL19, and CCL21. However, the egress process may be inhibited by some retention factors secreted by foam cells like Netrin-1 and semaphorin 3E. The whole process of monocytes entry and egress could be regulated by their biomechanical properties such as the membrane fluidity, the deformability, cell-cell junctions, and force of the membrane tether formation, as well as the surrounding biomechanical environment including the stiffness of the extracellular matrix, stretch from the neighboring vein, blood flow-induced shear stress, and hypertension. As for Ly6Clow monocytes, CCL5 plays a major role in their recruitments into the intima. Thereafter, the Ly6Clow monocytes differentiate into M2-type macrophages, which can phagocytose the apoptotic M1 macrophages and stabilize the plaque.
